Splitless injection
- Page ID
- 61207
Splitless injection is a variation of split injection and was designed particularly for trace analyses in highly diluted samples.
|
Features of splitless injection
|
|
Critical parameters of splitless injection
|
Splitless time
The splitless time is an important factor. The splitless time , i.e. the time the split exit is closed, should be long enough to allow transfer of the entire sample onto the column. It should, however, not be too long to avoid tailing of the solvent peak caused by traces of solvent vapour entering the column once the solvent film has already evaporated. In practice splitless times between 20 seconds and 1 minute are usually satisfactory.
Injection speed
In splitless injection, the injection speed differs considerably from direct injection and split injection. With the split injection it is important to inject as fast as possible in order to avoid dispersion of the injection band. Since the column temperature in splitless injection is lower than the boiling point of the solvent, solvent trapping will occur. Therefore the entire evaporation process and also the speed of evaporation have no effect on the width of the injection band. An injection may therefore be carried out slowly, hence improving quantification.
In a splitless injector, the entire vapourised sample should fit in the liner. Clearly this is easier if the sample is slowly injected into a wide-bore liner. Doing so, however, results in severe mixing of solvent vapour with carrier gas. This in turn complicates solvent recondensation in the column. For that reason, narrow-bore liners are used. The injection is slow to avoid explosion-like evaporation and turbulence that would result in mixing.
Split/splitless injector
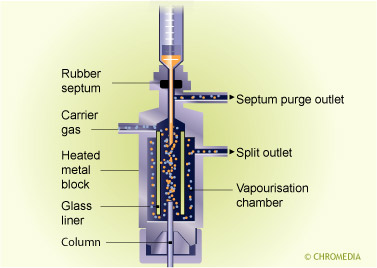
Splitless injection is carried out as follows:
- A sample is injected into a hot injector while the split exit is closed.
- The sample evaporates and is (almost) complly transferred onto the column.
- After some time (the splitless time) the splitter is opened.
- Any sample and solventy vapour present in the splitter is vented via the split opening. The oven remains cold during that time.
- After the splitter is opened, a temperature program is started, and the actual chromatographic separation starts.
Splitless mechanism

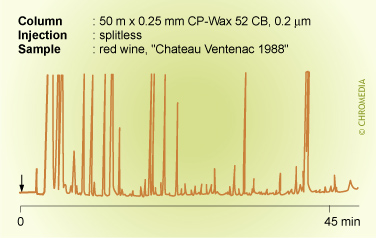
Splitless injection produces extremely sharp chromatographic peaks. This is especially surprising as the amount of solvent injected onto the column is many times larger compared to split injection.
Comparing splitless with split injection and assuming a commonly applied split ratio of 1 : 100, this means that with a closed splitter 100 times as much sample, and consequently also solvent, is introduced onto the column.
Problems with tailing of the solvent peak cannot be ruled out. The solvent peak is expected to have a very large tail. This tail is due to the presence of dead volumes in the liner and mixing of the solvent vapour with carrier gas in the dead volumes when the splitter is closed.
The problems encountered with the dead volume are remedied by opening the splitter after some time. The solvent that is still present in the injector and that could form the 'tail', is vented via the split opening. This is visible in the chromatogram as a sharply cut solvent peak. Typical splitless times are 20 to 60 s. This means that the input bands can yield up to 1 minute wide. Despite this fact, splitless injection can yield highly efficient chromatograms with peak widths of a few seconds only. This is due to concentration effects at the head of the column, resulting in the reduction of the broad injector band to a very small band. These effects are:
- Cold trapping , where the solvent does not play any part
- Solvent trapping, where the solvent plays the leading part
Splitless injection on CB phase