Introduction of Analyte
- Page ID
- 74969
What Happens in the Emission Source?
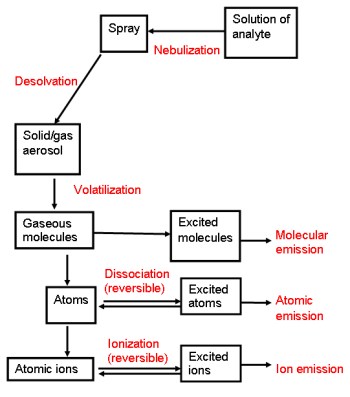
The composition of the analyte and matrix along with the emission source play a role in creating excited atoms that can give off an emission. THE MAJOR GOAL IS TO GET AS MANY ATOMS IN THE EXCITED STATE AS POSSIBLE WHILE MINIMIZING ALL THE OTHER PROCESSES. In the figure below, one can see that there are several factors that control the ability of atoms to go from the ground state to an excited state.
In following the flow chart working with a solution (not a solid), first the solution is nebulized (similar to a WindexTM bottle) and mist is produced. This mist can be desolvated to produce an aerosol of the analyte material. Volatization occurs via the source (flame, ICP, etc.) to produce gaseous molecules. From here, these molecules can break down into atoms or become excited molecules. The excited molecules can then give off an emission. The atoms can also become excited or form ions (via loss or gain of electrons). Lastly, the ions can have additional electrons that can be promoted to get excited ions.