In-class Questions: Ultraviolet/Visible Absorption Spectroscopy
- Page ID
- 96084
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
General aspects of UV/VIS absorption spectra
- Compare and contrast the absorption of ultraviolet (UV) and visible (VIS) radiation by an atomic substance (something like helium) with that of a molecular substance (something like ethylene).
- Do you expect different absorption peaks or bands from an atomic or molecular substance to have different intensities? If so, what does this say about the transitions?
- Compare a molecular absorption spectrum of a dilute species dissolved in a solvent at room temperature versus the same sample at 10K.
- Are there any other general processes that contribute to broadening in an absorption spectrum?
- Compare the UV absorption spectrum of 1-butene to 1,3-butadiene.
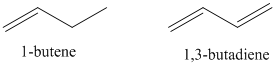
- Rank these from high to low energy.
- Compare the UV absorption spectrum of benzene and pyridine.
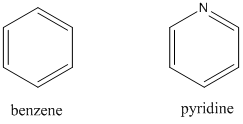
- The peaks in the 320-380 nm portion of the UV absorption spectrum of pyridine shift noticeably toward the blue (high energy) portion of the spectrum on changing the solvent from hexane (C6H14) to methanol (CH3OH). Account for this change.
- The peaks in the UV spectrum of benzene shift slightly toward the red (low energy) portion of the spectrum on changing the solvent from hexane (C6H14) to methanol (CH3OH). Account for this change.
UV/VIS spectroscopy as a qualitative and quantitative tool
- Is UV/VIS spectroscopy useful as a qualitative tool?
- Is UV/VIS spectroscopy useful as a quantitative tool?
- If you were using UV spectroscopy for quantitative analysis, what criteria would you use in selecting a wavelength for the analysis?
- What variables influence the recording of UV/VIS absorption spectra and need to be accounted for when performing qualitative and quantitative analyses?
- Provided the UV/VIS absorption spectra of HA and A– differ from each other, describe a method that you could use to measure the pKa of the acid.


