7.1: Ion-Selective Electrodes
- Page ID
- 81967
As the name implies, ion-selective electrodes are devices that are selective for a particular ionic species. The most well-known and highly used ion-selective electrode is a pH electrode, which is sensitive toward the H+ ion. A desirable aspect of ion selective electrodes is their ease of use. Using pH electrodes as an example, after appropriate calibration, it is quite trivial to measure the pH of a solution. pH meters are relatively small devices so that it is easy to transport a battery operated device to remote sites and measure the pH of samples like surface waters.
Ion-selective electrodes are used in a configuration where there is a completed circuit but the system is at equilibrium so there is no current flow. When there is no current flow it is possible to measure a potential. The basis of all ion-selective electrodes involves the measurement of a junction potential. The system is designed in such a way that the magnitude of a particular junction potential in the device depends only on the ion being measured. The design of a pH electrode will be discussed in detail and will exemplify the method by which other ion selective electrodes function.
7.1.1. pH Electrode
The components necessary for the measurement of pH involve internal and external Ag/AgCl reference electrodes and a thin glass membrane (about 50 um thick) (Figure 9). You are probably familiar with pH electrodes and have likely used one at some point to measure the pH of a solution. In all likelihood you used a single electrode system with a glass bulb on the bottom. In actuality, the device you used, and which is commonly in use today, is known as a combination electrode. The design of a combination electrode is shown in Figure 9. The system is an ingenious design that has the two Ag/AgCl reference electrodes incorporated into the single electrode system with appropriate connections to allow for a complete circuit.
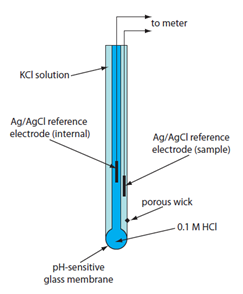
Figure 9. Diagram of a combination pH electrode.
(Figure from Analytical Chemistry 2.0, David Harvey, community.asdlib.org/activele...line-textbook/).
The inside of the glass membrane is filled with a solution consisting of 0.1 M hydrochloric acid (HCl) saturated with silver chloride (AgCl). The external layer of the glass membrane is immersed in the solution whose pH you wish to measure. The complete circuit of a pH electrode has multiple junction potentials, but only one – the potential between the glass membrane and the external solution whose pH is being measured – is important. All the other junction potentials in the system are constant or zero and combined into one cell constant. The membrane potential with the external solution varies with the concentration of H+ in the solution, and the two reference electrodes measure the potential difference across the glass membrane.
We need to look in more detail at the nature of the glass to understand how a variable membrane potential forms (Figure 10).
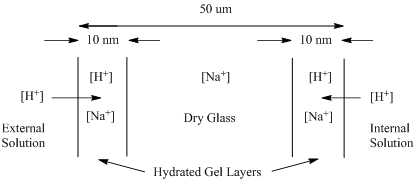
Figure 10. Diagram of the glass membrane in a pH electrode showing the dry glass and hydrated gel layers.
There are many ways to formulate glass. While glasses are based on a silicate framework (i.e., repeating SiO2 units), there are other impurities within the glass that give it different properties. The glass used in a pH electrode contains sodium (Na+) ions. When placed in contact with a solution, a microscopically thin (10 nm thick) hydrated gel layer forms on the surface of the glass. Within this gel layer, hydrogen ions from the solution can migrate a very short distance into the glass and displace sodium ions. The number of H+ ions that migrate into the gel layer depends directly on the concentration of H+ in the solution. For the internal solution, the concentration of H+ is constant so a fixed number of Na+ ions are displaced from the gel layer by H+ ions. For the external solution, the number of H+ ions changes with the pH so the number of Na+ ions displaced from the gel layer varies as a function of the pH. The ratio of [H+] to [Na+] in the outside gel layer influences the magnitude of the membrane potential. The reason the membrane potential varies with the ratio of the two ions is because the smaller H+ ions have a higher mobility than the larger Na+ ions.
It is important to note that a pH electrode does not inherently know the pH of a solution and must be calibrated against a buffer solution with a known pH. The electrode provides a measurement in mV and the measurement can be related to pH through an appropriate calibration. Therefore, the accuracy of pH measurements is only as reliable as the accuracy of the calibration buffers. Also, the electrode should be calibrated with a buffer that has a pH close to the pH you expect for the final solution. Common pH calibration buffers with pH values of 4, 7 and 10 are commercially available.
Another important consideration with a pH and any other ion-selective electrode is the possibility of interferences. Considering that H+ ions can migrate from the external solution into the hydrated gel layer, it should seem reasonable to conclude that other cations can similarly migrate into the gel layer and influence the junction potential. The degree to which other ions may influence the membrane potential depends on the nature of the glass and the concentration of the interfering ions. Many ion-selective electrodes come with instructions that list known interferences and the concentrations at which they become problematic to accurate measurements. For pH electrodes, values below pH 0.5 are unreliable because of what is known as an acid error. Values above pH 9 are less reliable because of alkaline error that is usually due to the relatively high concentration of other cations in solution that migrate into the glass.
The response of a pH electrode is also sensitive to the ionic strength of the solution. In solutions with exceptionally low ionic strength and low conductivity, the reading on a pH meter often takes a long time to stabilize after the pH electrode is inserted into the solution (rainwater is an excellent example of a solution with a low ionic strength – a pH measurement is a convenient way to monitor the acidity in acid rain). In solutions of higher ionic strength, the stabilization of the membrane potential and pH reading occur much faster. Some pH electrodes are designed purposely for the measurement of solutions with low ionic strengths. Such an electrode is designed to leak out some of the ions from the reference electrode to raise the ionic strength and conductivity of the external solution and promote faster establishment of the membrane potential.
Allowing the external gel layer of the glass membrane to completely dry out may prove detrimental to the performance of the electrode. pH electrodes are often capped for storage with a small volume of a pH 7 buffer in the cap. Before using a pH electrode removed from storage for measurements, it is advisable to soak it in a pH 7 buffer for several hours before use.
7.1.2. Other Glass Electrodes
Varying the composition of the glass enables the construction of ion selective electrodes that are sensitive for cations other than H+. Glass electrodes sensitive for Na+ and Ag+ are examples. When using these electrodes, one must always be concerned about interferences from other cations in solution and instructions with the electrode will describe possible interferences and the concentrations at which they become important.
7.1.3. Membrane Electrodes with Organic Polymers
In addition to membranes formed of glass, many membrane electrodes employ a hydrophobic organic polymeric material with appropriate functional groups designed to selectively associate with a certain ion. These functional groups can be anion exchangers, cation exchangers and neutral ionophores that are cavity compounds with the correct size to selectively bind certain metal ions. The ion can migrate into or bind to the polymeric membrane and the membrane potential depends on the concentration of that ion. A noteworthy feature of these membrane electrodes is that they can be designed to measure anionic species such as nitrate (NO3–) and chloride (Cl–) ions and cationic species such as NH4+ and Ca2+. As with other ion-selective electrodes, other anions may interfere with the measurement so care must be taken when using these devices.
7.1.4. Enzyme Electrodes
Enzyme electrodes are constructed similarly to the membrane electrodes described in the previous section except that the active material in the membrane is an enzyme. Typically the desired analyte reacts with the enzyme to produce a product whose concentration is monitored. A virtue of these electrodes is that they are highly selective for the particular substrate that binds to the enzyme. These electrodes are often constructed for use in clinical labs where there is a need to monitor a particular substrate on a regular basis as an indicator of a health issue. Examples of the use of an enzyme electrode are to measure urea or glucose.
7.1.5. Solid-State Electrodes
There are two common solid-state ion-selective electrodes where the active surface consists of a polished crystal of lanthanum fluoride (LaF3) or silver sulfide (Ag2S). The electrode is sensitive toward either of the ions that make up the crystal. While there is seldom interest in measuring the concentration of lanthanum, fluoride is often added to municipal water supplies to reduce tooth decay. Fluoride selective electrodes are easy to use on a sample by sample or continuous monitoring basis to insure that the fluoride levels of the water are in the proper range. Similarly, there is usually not much need to measure silver ion in solution but sulfide ion is indicative of the ability to form hydrogen sulfide (H2S), a toxic, foul-smelling gas. The silver sulfide electrode can also be doped with cadmium, copper and lead to create ion-selective electrodes for these metals.
7.1.6. Gas-Sensing Electrodes
Gas-sensing electrodes are designed to measure water-soluble gases such as carbon dioxide (CO2), sulfur dioxide (SO2), nitrogen dioxide (NO2), ammonia (NH3). The electrode has a semi-permeable membrane that allows the gas of interest to pass through it into an internal solution. These gases can all react with water to form acids that liberate hydronium ions (CO2, NO2 and SO2) or a base that liberates hydroxide ions (NH3). The electrode incorporates a pH electrode in contact with the internal solution and the measured pH can be related back to the amount of gas in solution.


