5. Electrochemical Cells
- Page ID
- 81962
Before developing analytical methods based on electrochemistry, it is worth exploring aspects about electrochemical cells. Concepts needed to comprehend the nature of an electrochemical cell are informative in understanding some of the analytical methods we will develop. From a more practical standpoint, batteries are examples of electrochemical cells.
Describe what you know about an electrochemical cell.
The components of an electrochemical cell are shown in Figure 7.
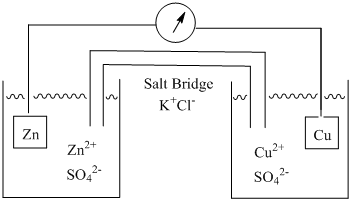
Figure 7. Diagram of the components in an electrochemical cell.
The particular cell shown involves a half reaction with zinc and a half reaction with copper.
\[\mathrm{Zn^{2+}(aq) + 2e^- = Zn(s) \hspace{40px} E^o = -0.763\: V}\]
\[\mathrm{Cu^{2+}(aq) + 2e^- = Cu(s) \hspace{40px} E^o = 0.337\: V}\]
Based on the two Eo values, the copper ion will be reduced and zinc metal will be oxidized. In an electrochemical cell, the reduction half reaction is referred to as the cathode and the oxidation half reaction is referred to as the anode. By convention, the anode is always put on the left and the cathode on the right in the diagram.
The zinc half-cell consists of a piece of zinc metal in a solution containing zinc ion. The copper half-cell consists of a piece of copper metal in a solution containing copper ion. If a half reaction does not form a solid metallic species (e.g., Fe3+ + e– = Fe2+) an inert metal such as platinum is used in the cell.
The two half-cells need to be connected to complete the circuitry and allow the reaction to proceed. Two connections are needed for a complete circuit. One is a metal wire that connects the two pieces of metal. The other is something known as a salt bridge that connects the two solutions.
What processes are responsible for conduction of electricity in an electrochemical cell?
The processes responsible for the current flow in an electrochemical cell depend on which part of the cell you are in. For the metallic components (zinc, copper, copper connecting wire), electrons are responsible for the current flow. In the solution, conduction of electricity is caused by migration of ions.
The ability of ions to conduct electricity is the reason why someone should never use a hairdryer while sitting in a bathtub full of water. If a hairdryer is dropped into the water, the water conducts electricity because of ions in it with the end result that the person will be electrocuted. Conductivity is a measurement of the ability of a solution to conduct electricity. The conductivity of a solution directly correlates with the ionic strength of the solution. Many science buildings have a device that is designed to generate highly purified water. One of the goals of these purification systems is to deionize the water. With these systems the conductivity is measured to determine the degree to which the water has been deionized (the reading is reported as a resistance and the higher the resistance, the less conductive the solution).
It is also important to consider the portions of the cell where the metal interfaces with the solution. In the cathode where reduction occurs, electrons must “jump” from the metal to a species in solution. In the anode of the cell represented in Figure 7, zinc atoms need to give up two electrons and a zinc ion is released into the solution. For an anodic half-cell with two water-soluble species (e.g., Fe2+ = Fe3+ + e–), an electron would need to “jump” from a species in solution to the platinum electrode.
What is the purpose of the salt bridge?
In order to understand the purpose of the salt bridge it is necessary to consider the process taking place in each of the half cells in Figure 7. If each half cell started at standard state conditions, the cathode would begin with a 1 M concentration of a copper salt such as copper sulfate ([Cu2+] = 1 M; [SO42-] = 1 M) and the anode would have a zinc salt such as zinc sulfate ([Zn2+] = 1 M; [SO42-] = 1 M). Note that in both half cells, the sulfate ion is a spectator ion that is not involved and does not change in the electrochemical reaction. As the electrochemical reaction proceeds, Cu2+ in the cathode gets reduced and plates out as copper metal. In the other half call, zinc metal gets oxidized to form Zn2+. Without any form of intervention, this means that over time [Cu2+] < [SO42-] in the cathode and [Zn2+] > [SO42-] in the anode. The buildup of charge in both of the half cells is an undesirable situation because nature wants to maintain systems that are neutral. If this charge continued to build up, it will inhibit the electrochemical reaction and prevent it from going to its full extent. The purpose of the salt bridge is to act as a source of spectator ions that can migrate into each of the half cells to preserve neutrality. Any charge buildup in the solutions of the two half cells is known as a junction potential. Therefore, the purpose of a salt bridge is to reduce the junction potential between the solution interface of the two half cells.
What would you put inside a salt bridge?
First, it is important to put ionic species into the salt bridge that will not be reduced or oxidized in either of the half cells. Alkali cations and halide anions would be ideal for this purpose. It is also important that the charge balance in each of the half cells facilitated by the ions in the salt bridge occurs at the same rates. That means that the halide anions moving from the salt bridge into the anode to balance out the excess Zn2+ ions do so at the same rate as the alkali cations moving from the salt bridge into the cathode to balance out the depletion of Cu2+ ions. Ions have a property known as mobility and the mobility of an ion depends on its size. Smaller ions have a higher mobility than larger ions. That means that the ideal species for a salt bridge should have a cation and anion of the same size and charge. Potassium chloride is the ideal species for incorporation into a salt bridge, as K+ and Cl– have the same number of electrons and are approximately the same size. Potassium nitrate (K+NO3–) can also be used in a salt bridge. Amazingly, the nitrate ion, which has atoms with second shell electrons, has approximately the same size as a chloride ion, which has atoms with third shell electrons.
Another thing to consider is the concentration of KCl in the salt bridge. It is desirable to have a salt bridge that can overcome the possibility of a large charge buildup. To achieve this and not deplete the ions in the salt bridge over the course of the reaction, the KCl is typically at a high concentration, usually 4 M.
Describe two types of situations that would result in the irreversibility of an electrochemical process
An interesting aspect of an electrochemical cell is that it can be operated in two directions. If the circuitry is completed and the reaction proceeds in its spontaneous direction toward equilibrium, it is referred to as a voltaic or galvanic cell. In this case a current is drawn from the cell and can be used to perform some sort of work (e.g., light a bulb in a flashlight or operate a cell phone). At some point the cell or battery will “die” as the reaction reaches equilibrium and no more current can be drawn from it. Next time your car battery dies, you may feel better about the situation by remembering that it has just reached equilibrium.
If the cell instead is attached to an external source of power (e.g., plugged into a wall outlet), the reaction can be forced it its reverse direction back away from equilibrium. This is what happens when a battery is recharged. Rechargeable batteries require the use of a reversible reaction. An electrochemical cell being forced in its non-spontaneous direction is referred to as an electrolytic cell.
There are two situations that factor into the reversibility of reactions used in electrochemical cells. One involves chemical reversibility, which relates to the stability of the reactants and products. The other involves electrochemical reversibility, which involves the kinetics of electron transfer and relates to the ability to regenerate or recharge the cell to its initial conditions.
One common misconception is that an electrochemical reaction that produces a gas such as the reduction of hydrogen ion to hydrogen gas is chemically irreversible because the gas escapes.
\[\mathrm{2H^+(aq) + 2e^- \rightarrow H_2(g)}\]
However, if the cell is designed properly and is sealed, the gas can be trapped and reversing the potential through the use of an external power source can drive the reaction in the reverse direction. Therefore, electrochemical reactions that produce a gas are not necessarily chemically irreversible.
An example of an electrochemical process that is chemically irreversible occurs if the product rapidly decomposes to something else. In this case, when an external power source is applied to reverse the process, the appropriate species is no longer in solution. In the example below, if the A– species degrades rapidly to B– and C, there is no remaining A– for the chemical regeneration of A.
\[\ce{A + e^-} \rightleftharpoons \ce{A^-} \rightarrow \ce{B^- + C}\]
The second example of an irreversible electrochemical reaction occurs when there is something known as an overvoltage or overpotential. Since electrons must transfer from one species to another in an electrochemical reaction, the kinetics of the electron transfer must be considered. In cases of slow kinetics, it is possible to have an electrochemically irreversible reaction.
If we consider the reduction of H+ to hydrogen gas shown above, there is the key step where the electron must “jump” from the electrode to the hydrogen ion in solution. With some electrochemical reactions, there is a resistance of the electron to making the jump. If one were to apply a potential that in theory was suitably large such that the electrons should complete the jump, it would still not happen. The electron can be forced to “jump” by applying a higher voltage – an overpotential – to the electrode (electrons of higher energy are put onto the electrode until a point is reached where it becomes favorable for an electron to leave the electrode and go to the ion in solution). You are likely familiar with the concept of activation energies in chemical reactions. The occurrence of an overpotential indicates the presence of an activation energy barrier for an electrochemical reaction.
Whether or not a particular half reaction has an overpotential is determined in part by the nature of the electrode material. The reduction of hydrogen ion to hydrogen gas has almost no overpotential with a platinum electrode but has a very high overpotential with mercury and many other electrodes.
As an aside, it is worthwhile to examine two particular half reactions that have practical applications and potential future implications for society. These two reactions are shown below.
\[\mathrm{2H^+(aq) + 2e^- = H_2(g) \hspace{40px} E^o = 0.00\: V}\]
\[\mathrm{O_2(g) + 4H^+(aq) + 4e^- = 2H_2O \hspace{40px} E^o = 1.229\: V}\]
Based on the Eo values, the spontaneous reaction involves the oxidation of hydrogen gas and reduction of oxygen gas, as shown in the balanced reaction below.
\[\mathrm{2H_2(g) + O_2(g) = 2H_2O \hspace{40px} E^o = 1.229\: V}\]
A device that electrochemically combines hydrogen and oxygen gas and uses the transfer of electrons as a source of electricity is known as a fuel cell. One example of the use of this technology as a source of electricity is aboard the International Space Station.
If one considers the reverse reaction shown below, through the application of an external source of power it will be possible to electrochemically convert water to hydrogen and oxygen gas.
\[\mathrm{2H_2O = 2H_2(g) + O_2(g)}\]
The intriguing aspect of the electrolytic splitting of water into hydrogen and oxygen gas is that hydrogen is a useful fuel for a fuel cell or through combustion. If someone were able to economically carry out this reaction, it would mean that our fuel would come from water, thereby providing a limitless source of fuel. Also, because the reaction is best done under conditions with a relatively high ionic strength, it could be done using ocean water.
There are two problems with economically carrying out the electrolytic splitting of water. One is that it takes energy to do it, since it’s the reverse of the spontaneous direction. Because of losses of efficiency when combusting hydrogen, it would take more energy to split water then you would get in return by using hydrogen as a fuel. A second problem is that both half reactions have overpotentials with many different electrodes meaning that it will cost even more to carry out the reaction.
An active area of research is an attempt to devise electrodes that have two particular features. One is that they are made of materials that do not have high overpotentials toward the two relevant half reactions (Note that two different electrodes are needed: one for the hydrogen half reaction and the other for the oxygen half reaction). The second feature would be a system where sunlight could be used as a source of power to assist the splitting reaction. In the two electrodes, the energy from the sun would be used to promote an electron on the electrode into a higher molecular orbital. With that extra energy it would be much easier for the electron to transfer to the species in solution and then much less costly to split the water. Many of the electrodes being examined in this application are transition metal complexes and while some advances have been made, it is also critical that the electrode be inexpensive enough to make the entire process cost effective. No one has yet to find electrodes with low enough overpotentials and the ability to harness the energy of the sun to facilitate the reactions in a cost effective manner.
Shorthand Notation for an Electrochemical Cell
There is a shorthand notation used to specify the conditions of an electrochemical cell. The notation shows the anode on the left and cathode on the right. The concentrations of important species in each of the half reactions are included. Spectator ions are not included in the notation. Phase boundaries are shown with a single line (|) and a salt bridge with a double line (||). The electrochemical cell described above would have the following notation:
\[\ce{Zn \:|\: Zn^2+ (1\: M) \:||\: Cu^2+ (1\: M) \:|\: Cu}\]


