In-class Problems
- Page ID
- 81725
In-class Questions – Electrochemistry
- Define what is meant by oxidation and reduction.
- Define what is meant by an oxidizing and reducing agent. Give a good example of each.
- Define what is meant by a half-reaction.
- Give an example of a half-reaction and determine whether a half-reaction can be an equilibrium expression. Why or why not?
Chemical Energy
Now let's think about chemical energy (otherwise known as the Gibbs energy and denoted by G). A bottle of pure A has some amount of chemical energy. A bottle of pure B has some amount of chemical energy. A solution of A in water that is 2 Molar has some amount of chemical energy as does a solution of B in water that is 2 Molar.
- Do you think that those four examples have the same or different chemical energy?
- How would you measure or determine the absolute chemical energy of those four systems?
- \[\mathrm{A \Leftrightarrow B}\]
When will the chemical energy of that system achieve its lowest value?
- \[\mathrm{[A] + [B] = 2\: Molar}\]
is always satisfied, and the reaction of A to produce B has a fairly large equilibrium constant.
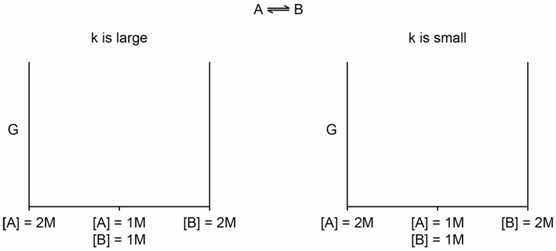
- What would the plot look like if the reaction had a fairly small equilibrium constant?
- Why do we need to define something called the standard state?
- What does it mean for the difference in chemical energy (DG) to be positive or negative?
Electrochemical Cells
- Describe what you know about an electrochemical cell.
- What processes are responsible for conduction of electricity in an electrochemical cell?
- What is the purpose of a salt bridge? What would you put inside a salt bridge?
- Describe two types of situations that would result in the irreversibility of an electrochemical process.
Use of the Nernst Equation
Potassium dichromate reacts with Fe(II) to produce Cr(III) and Fe(III).
- What is the standard state potential and K for this reaction?
- What is the cell potential if one half-cell is made up with 1.50 M potassium dichromate and 0.30 M chromium(III)nitrate hexahydrate in 1.00 M nitric acid and the other half cell is made up with 0.050 M iron(III)chloride hexahydrate and 0.10 M iron(II)chloride tetrahydrate?
- What is the cell potential if the chromium half-cell were operated at a pH of 7 instead of using 1 M nitric acid?
Electrodeposition/Electrogravimetry
Will the presence of Fe(II) at 0.0800 M interfere with the electroplating of 99.9% of the cadmium(II) in a solution in which the cadmium is expected to be present at a concentration of no less than 0.0500 M? Calculate potential values relative to a standard hydrogen electrode.
Suppose the solution had 0.0800 M Cr(III) as a possible interference. Is it possible to plate out 99.9% of the cadmium(II) without any interference from the chromium.
Coulometry
- Draw the plot you would obtain for current (y-axis) versus time (x-axis) if you applied a constant potential high enough to carry the reduction of Cd(II) to cadmium metal. The system has an electrode with a large surface area.
- How would you relate the outcome of your plot above to the concentration of Cd(II) in the sample?
- What advantages does coulometry have over electrogravimetry?
Coulometric Titration
What are some advantages of using a coulometric titration?
Amperometric Titration
Draw the plot that would be obtained in an amperometric titration if each of the following occurred (assume that the system is set up with a reducing potential):
- Only the analyte undergoes a reduction at the applied potential.
- Only the titrant undergoes a reduction at the applied potential.
- Both the analyte and titrant undergo a reduction at the applied potential.
Potentiometric Titration
Consider a solution of iron(II) that is to be titrated with cerium(IV). The products of the reaction are iron(III) and cerium(III). The course of the titration will be monitored potentiometrically.
\[\mathrm{Fe(II) + Ce(IV) = Fe(III) + Ce(III)}\]
If 20 ml of an 0.10 M solution of Fe(II) is to be titrated with 0.10 M Ce(IV), calculate the junction potential that would be measured at a platinum electrode when 5 ml, 10 ml, 20 ml, and 30 ml of titrant have been added.
The following steps will allow you to work through the calculation of the junction potentials.
- Write an expression for the total electrochemical potential of this system (this is the Nernst equation for the complete reaction).
- What is the total electrochemical potential of the titration reaction at any point during the titration? Think carefully about this because your first intuition may not be correct.
- Which half reaction is easier to use to calculate the junction potential before the equivalence point? Which half reaction is easier to use after the equivalence point?
Voltammetric Methods
- How can electrostatic migration be eliminated in an electrochemical cell?
- Samples subjected to voltammetric methods are usually purged with nitrogen or another inert gas before the analysis and maintained under an inert atmosphere during the analysis. Why is this done?
Anodic Stripping Voltammetry
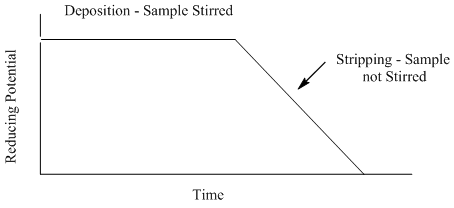
- What feature of the plot can be related to the concentration of the metal?
- What advantage(s) does anodic stripping voltammetry offer over coulometry or electroplating?
Linear Sweep Voltammetry

- What feature of the plot could be related to concentration?
- What feature of the plot can be used for species identification?
- For the exact same solution of Cd(II) and Zn(II), draw the current that would be observed if the solution is not stirred.
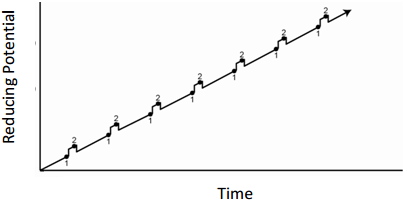
- What advantage(s) does the method in question 5 have over regular linear sweep measurements?
Cyclic Voltammetry

- Draw a plot that could be obtained for an electrochemical reaction that is chemically irreversible and forms an electrochemically inactive product.
- Draw the plot of current vs. potential that would be obtained for a reversible chemical reaction in which only the reverse reaction has an overpotential, but the potential eventually is sufficient to complete the reverse reaction.
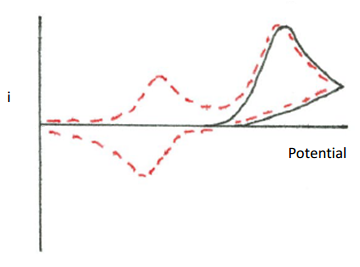
- Propose a reaction mechanism that would explain the following cyclic voltammogram. The first voltage cycle is shown as a solid line. The second voltage cycle is shown as a dotted line. A third voltage cycle gives the same output as the dotted line. Assume that a reducing potential is applied.