24.5: Biological Amines and the Henderson-Hasselbalch Equation
- Page ID
- 239379
Objectives
- identify the form that amine bases take within living cells.
- use the Henderson‑Hasselbalch equation to calculate the percentage of a base that is protonated in a solution, given the pKa value of the associated ion and the pH of the solution.
- explain why organic chemists write cellular amines in their protonated form and amino acids in their ammonium carboxylate form.
The Henderson-Hasselbalch equation is a very useful equation relating the pKa of a buffered solution to the relative amounts of an acid and its conjugate base. In Section 20-2, we used the Henderson-Hasselbach equation to show that under physiological pH, carboxylic acids are almost completely dissociated into their carboxylate ions.
The Henderson-Hasselbalch equation:

So, what does the side chain of an lysine amino acid residue look like if it is on the surface of a protein in an aqueous solution buffered pH 7.0? Is it protonated or deprotonated? The values in the Henderson-Hasselbalch can be used for an amine with the ammonium salt as, HA = RNH3+, and the amine as being, A- = RNH2. With an approximate pKa of 10.8, it should be >99% protonated, in the positively-charged, ammonium form:
7.0 = 10.8 + log ([RNH2] / [RNH3+])
([RNH2] / [RNH3+]) = 1.6 x 10-4
. . . so, [RNH3+] >> [RNH2]
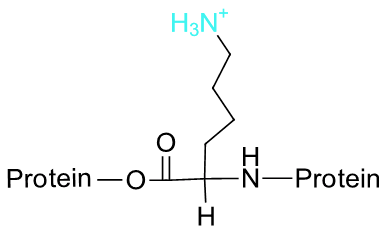
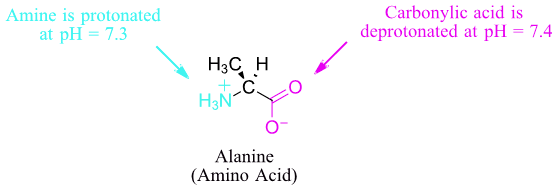
So, in an aqueous solution buffered at pH 7, carboxylic acid groups can be expected to be essentially 100% deprotonated and negatively charged (ie. in the carboxylate form), and amine groups essentially 100% protonated and positively charged (i.e., in the ammonium form). Alcohols are fully protonated and neutral at pH 7, as are thiols. The imidizole group on the histidine side chain has a pKa near 7, and thus exists in physiological solutions as mixture of both protonated and deprotonated forms.
Exercise \(\PageIndex{1}\)
Would you expect an aromatic hetererocycle, pyrrole, to be protonated at pH = 7.3? Use the Henderson-Hasselbalch equation to determine your answer. pKa of imiazole is 0.4.
- Answer
-
7.3 = 0.4 + log ([RNH2] / [RNH3+])
([RNH2] / [RNH3+]) = 7.9 x 106
. . . so, [RNH2] >> [RNH3+] so pyrrole would be almost completely unprotonated at pH = 7.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

