23.11: Carbonyl Condensations with Enamines - The Stork Reaction
- Page ID
- 239369
Objectives
After completing this section, you should be able to
- write an equation to illustrate the three‑step Stork enamine reaction.
- write a detailed mechanism for each of the three steps of the Stork enamine reaction.
- identify the product formed, and the various intermediates (i.e., the enamine, the Michael‑type adduct), in a given Stork enamine reaction.
- identify the reagents needed to synthesize a given compound by a Stork enamine reaction.
Key Terms
- Stork enamine reaction
Study Notes
If we try to use a monoketone as a donor molecule in a Michael reaction, we will obtain a poor yield. An alternative route to the product that would be expected from such a synthesis is via the Stork enamine reaction. You may wish to review the formation of enamines (from ketones and secondary amines) before you proceed with this section; if so, review Section 19.8.
Synthesis of Enamines
As previously seen in Section 19.8, aldehydes and ketones react with 2o amines to reversibly form enamines. Enamines can add to acid halides to form 1,3-diketones and α, β-unsaturated Michael acceptors to from 1,5-dicarbonyls.
Example
Reversibility
Typically the Following 2o Amines for Enamine Reactions
Enamines have resonance structures similar to enolates. The lone pair electrons on the nitrogen are conjugated with the double bond and can donate electron density to the α-carbon. This allows the α-carbon of enamines to be nucleophilic in much the same fashion as enolates. The increased electron density of the α-carbon enamine, N,N-Dimethylaminoethylene, is shown as a yellow/green color in its electrostatic map. This is analogous to the increased electron density of the α-carbon of an enolate.
Enamines act as nucleophiles in a fashion similar to enolates. Because of this enamines can be used as synthetic equivalents as enolates in many reactions. This process requires a three steps: 1) Formation of the enamine, 2) Reaction with an electrophile to form an iminium salt, 3) Hydrolysis of the iminium salt to reform the aldehyde or ketone. Some of the advantages of using an enamine over an enolate are enamines are neutral, easier to prepare, and usually prevent the overreaction problems plagued by enolates. Also, enamine are more effective at the Michael reaction compared to a mono-carbonyl enolate.
Acylation of Enamines
Example
Mechanism
The mechanism starts with the formation of an enamine. The enamine adds to the electrophilic carbonyl carbon of the acid halide to form an iminium bond with tetrahedral alkoxide as an intermediate. The alkoxide reforms the carbonyl bond and eliminate a halide anion as a leaving group. The iminium bond is then hydrolyzed to reform the carbonyl to create a 1,3-dicarbonyl compound as the product of a nucleophilic acyl substitution.
1) Formation of the enamine
2) Nucleophilic attack
3) Leaving group removal
4) Reform the carbonyl by hydrolysis
Michael Reaction Using Enamines: The Stork Enamine Synthesis
Enamines add to α, β-unsaturated carbonyls in a Michael-like reaction. The net reaction is the addition of a ketone to a α, β-unsaturated carbonyl to product a 1,5 dicarbonyl compound as the end product. This reaction is commonly known as the Stork enamine reaction after Gilbert Stork of Columbia University who originated the work.
Example
Mechanism
After formation, the enamine adds to the electrophilic alkene carbon of the a α, β-unsaturated carbonyl form an iminium bond. The pi electrons of the alkene are pushed onto the oxygen through conjugation to form a tetrahedral alkoxide as an intermediate. Protonation of the alkoxide form an enol which undergoes tautomerization to reform the ketone. In the last step of the mechanism, the iminium bond is hydrolyzed to reform the carbonyl to create a 1,5-dicarbonyl compound as the product of a Michael-like reaction.
1) Formation of the enamine
2) Nucleophilic attack on the carbon β to the carbonyl
3) Protonation
4) Tautomerization
4) Reform the carbonyl by hydrolysis
Planning a Synthesis Using the Stork Enamine Synthesis
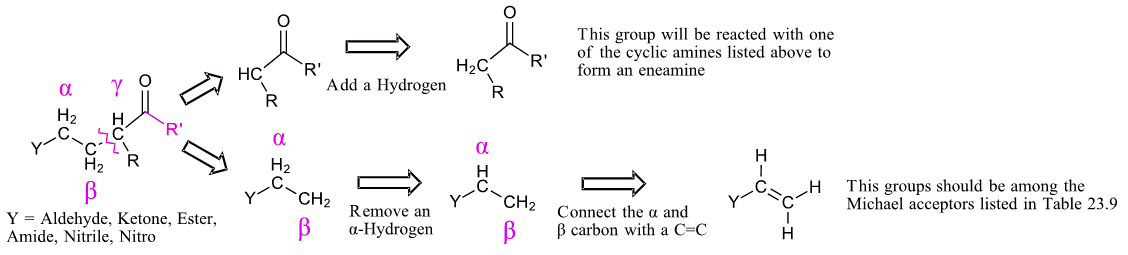
Planning a synthesis using a Stork enamine reaction is very similar to that of a Michael reaction (Section 23-10). A target molecule can possibly be made using a Stork enamine reaction if it contains a 1,5-dicarbonyl. Like the Michael reaction, the key bond cleavage is a C-C bond between beta and gamma carbons from a carbonyl-like group. The fragment with the Y group loses an alpha-hydrogen and then forms a C=C bond between the alpha and beta carbon. The carbon of the other fragment gains a hydrogen. This fragment should possess an acidic alpha-hydrogen and should be made up of Michael donor fragments listed in Table 23.9.
Worked Example
How could the following molecule be made using a Michael Reaction?
- Answer
-
Pathway 1

Solution
Exercises \(\PageIndex{1}\)
1) Draw the product of the reaction with the enamine prepared from cyclopentanone and pyrrolidine, and the following molecules.
a)
b)
c)
2) What would be the starting materials necessary to make the following molecules during a Stork enamine reaction.
a)
b)
- Answers
-
1)
a)
b)
c)
2)
a)
b)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

