5.3: Use of the Reaction With Bio Derived Molecules
- Page ID
- 306500
Example of use with a bio-based molecule
Chosen Article Title: "Catalytic Hydrogenation of Arenes in Water Over In Situ Generated Ruthenium Nanoparticles Immobilized on Carbon"
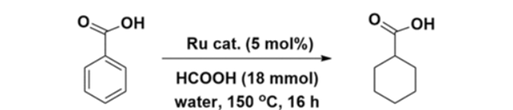
The purpose of the study was to find the most efficient way to generate Ruthenium Nanoparticles especially those with aromatic rings attached to them and how to neutralize them when in the presence of water. Due to that the particles are used for polymer membranes, electrochemical capacitors, and coatings for many different plastics and metals. The bio-based molecule that the study focused on using was mostly Benzene Complexes of Ruthenium(II) which is Ruthenium surrounded by aromatic rings.
The study found that the most efficient catalytic system used cooperative synergy of [(η6‐benzene)Ru(ethylenediamine)Cl]+ and formic acid to best immobilize the carbon in water. This showed that the found metal used in their Catalytic Hydrogenation step was the most successful at decreasing any carbon found in the water.
References
Dwivedi, A. D.; Rai, R. K.; Gupta, K.; Singh, S. K. Catalytic Hydrogenation of Arenes in Water Over In Situ Generated Ruthenium Nanoparticles Immobilized on Carbon. ChemCatChem 2017, 9 (11), 1930–1938.
Contributors
Alexander Towle

