5.4: Chromatography
- Page ID
- 84216
Learning Outcomes
- Define chromatography.
- Distinguish between the stationary and mobile phases.
- Explain how components of a substance are separated based on attraction to each phase.
When a pure substance is mixed with another pure substance in which it is soluble, the substances become completely interspersed at the molecular level. In thinking about making solutions at the molecular level an analogy to a can of marbles may be useful. In the analogy, a layer of red marbles is placed in the bottom of a can and covered with a second layer of white marbles. After shaking the can for a short time, the marbles are mixed randomly.
Now let us imagine that you want to collect all the red marbles again. If you simply shake the can, it is unlikely that you will ever divide the marbles into two layers, each with only one kind of marble. Similarly, if two miscible liquids are combined, a chemist cannot simply un-mix the liquids into pure components.
Continuing the analogy, what if a few green marbles and blue marbles are placed into the can? Given enough red and white marbles, it may be difficult to determine that the green marbles and blue marbles are actually there. Similarly, when chemists have a multi-component solution which may contain traces of important chemical species, they are faced with the challenge of detecting whether these chemicals are present in solution.
To deal with these difficulties, chemists employ different methods to separate solutions into their components. Two essential techniques are distillation and chromatography.
Another useful set of techniques for separating mixtures is called chromatography. Perhaps the simplest of these techniques to describe is paper chromatography, an example of which is shown in the video below.
Video: Simple paper chromatography. A simple demonstration on paper chromatography using marker ink and water
Three substances are applied to a strip of chromatography-grade paper (the stationary phase of this experiment). As the liquid level rises and meets the spots, the sample partially dissolves in the liquid (the mobile phase because it is moving) and travels up the plate within the solution. Different substances will travel different distances along the plate. The distance that a substance will travel depends on how strongly it adheres to the stationary phase (a process called adsorption) versus how much time it spends dissolved in the mobile phase.
The more a substance adsorbs, the less it dissolves and the less it moves along the plate. The pink and blue spots at the end of the video are examples of substances highly adsorbed to the stationary phase. The less a substance adsorbs, the more it dissolves and the farther it travels, such as the yellow on the far left. The process is continued until a good separation is created. In this manner, a mixture of substances may be separated such as the middle sample, which was originally green but separated into blue and yellow. Notice that while it was not initially obvious that the middle spot contained both substances, this fact is clear after performing paper chromatography.
All forms of chromatography work on the same general principle as paper chromatography. There is always a stationary phase which does not move and a mobile phase which does. The various components in the mixture being chromatographed separate from each other because they are more strongly held by one phase or the other. Those which have the greatest affinity for the mobile phase move along the fastest.
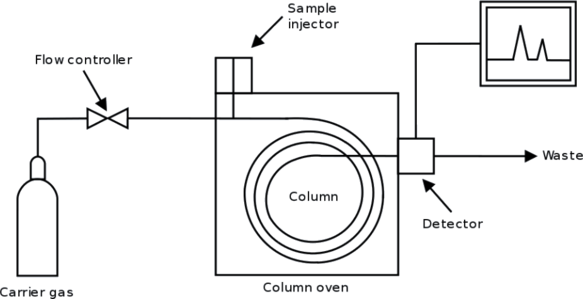
The most important form of chromatography is gas chromatography or vapor-phase chromatography. A long column is packed with a finely divided solid whose surface has been coated with an inert liquid. This liquid forms the stationary phase. The mobile phase is provided by an inert carrier gas, such as He or N2, which passes continuously through the column (seen below), among the solid particles. A liquid sample can be injected into the gas stream at the sample injector and vaporized just before it enters the tube. As this sample is carried through the column by the slow stream of gas, those components which are most soluble in the inert liquid are held up, while the less-soluble components move on more rapidly. The components thus emerge one by one from the end of the tube, into the detector. In this way it is possible to separate and analyze mixtures of liquids which it would be impossible to deal with by distillation or any other technique.
The development of chromatography is one of the major revolutions in technique in the history of chemistry, comparable to that which followed the development of an accurate balance. Separations which were previously considered impossible are now easily achieved, sometimes with quite simple apparatus. This technique is particular essential to the science of biochemistry, in which complex mixtures are almost always encountered. In the field of environmental chemistry, chromatography has helped us separate and detect very low concentrations of contaminants like DDT or PCB (polychlorinated biphenyls). The major drawback to chromatography is that it does not lend itself to large-scale operation. As a result it remains largely a laboratory, rather than an industrial, technique for separating mixtures.

