The aldol reaction
- Page ID
- 25540
Condensation vs. Addition

Enolate Geometry in Aldol Additions
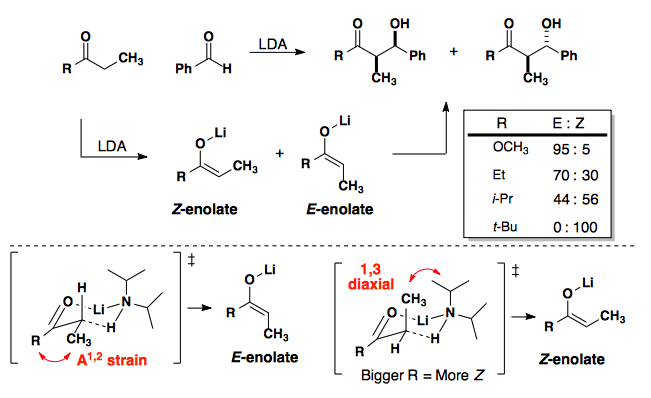
Zimmerman-Traxler Model
Zimmerman proposed a chair-like geometry for the Ivanov Reaction (a variant of the aldol reaction).
J. Am. Chem. Soc., 1956, 79, 1920-1923.
This model has since been generalized to the aldol reaction and is now known as the Zimmerman-Traxler Model.
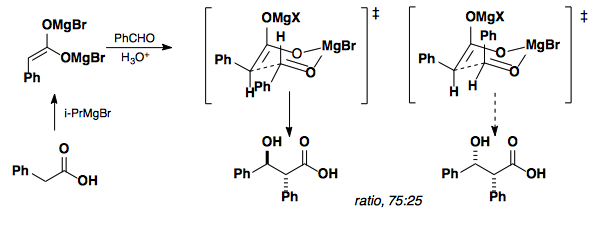
As can be seen from the above data, the model does not translate well to all enolates and works best when X is a large group; however, optimizations of the metal center have allowed for the development of a general model.
Boron aldol reaction
Switching to a Boron Lewis acid provides several advantages over other lewis acids.
The shorter B–O bonds create a tighter transition state thus destabilizing the disfavored transition state.
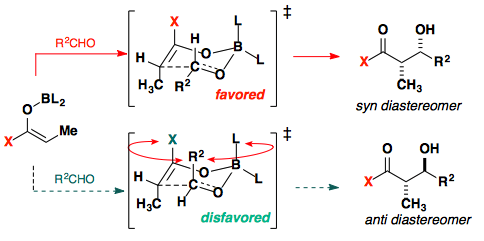
Boron lewis acids have been developed to provide either Z or E enolates in high selectivity.
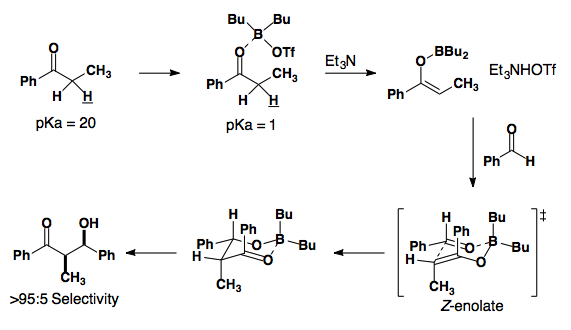
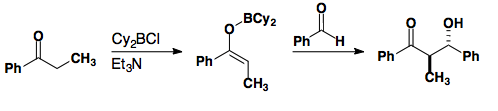
Ten years later, conditions were developed to provide the E-enolate in high selectivity.
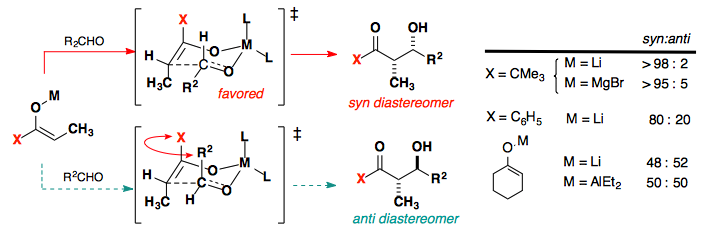
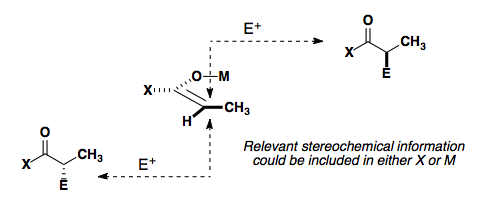
Absolute stereochemistry
With good methods to reliably control the diastereoselectivity, we will now look at methods to control the facial selectivity of the reaction in order to control the absolute stereochemistry of the reaction.
Control of the reaction can original from a Chiral X group, such as the Evans auxiliary, or from a chiral ligand attached to the metal center.
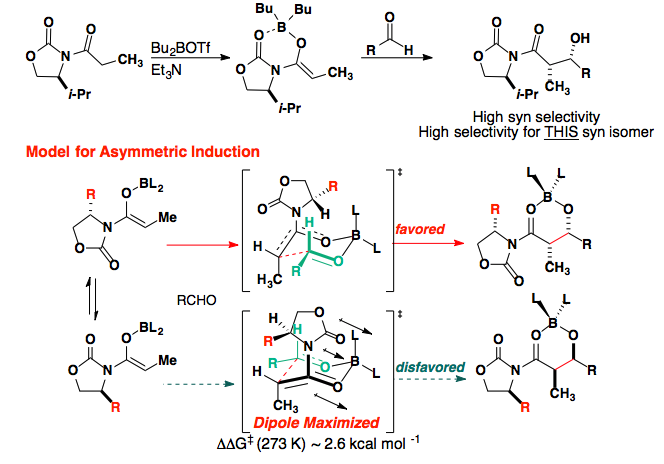
J. Am. Chem. Soc., 1981 ,103, 2127-2129.
The enolate substituent also plays a large role in the selectivity.
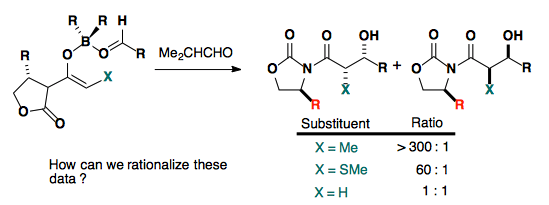
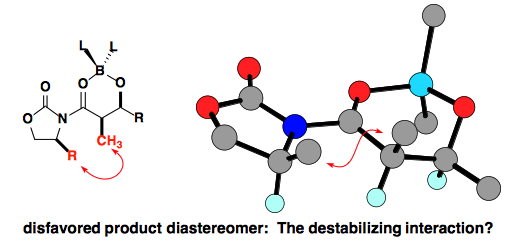
This is believed to arise from a destabilizing interaction in the product.
Anti-selectivity
Anti-selectivity can be acheived in several ways. Switching to a different auxiliary allows for the formation of the E-enolate.
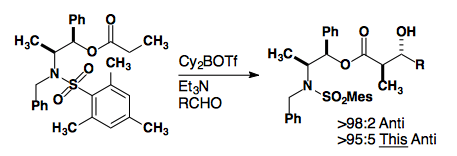
J. Am. Chem. Soc., 1997, 119, 2586-2587.
Alternatively, the Evans Auxiliary can generate the anti-product by switching to a magnesium lewis acid. This reaction is believed to proceed through a boat transition state to provide the anti-diastereomer.
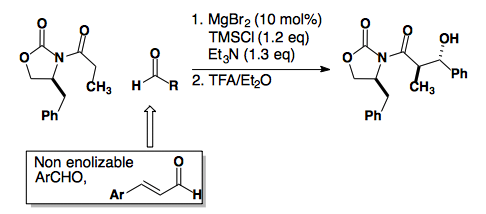
J. Am. Chem. Soc., 2002, 124, 392-393.
Examples
Prelog-Djerassi Lactone
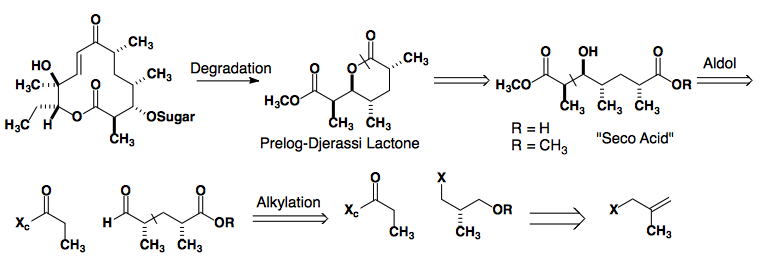
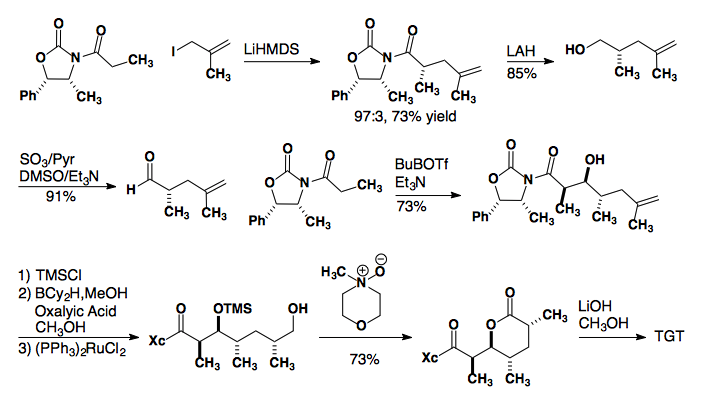
Thiazolidinethione Auxiliary

J. Org. Chem., 2001, 66, 894-902.
Acetate Aldol
Several oxazolidinone derived auxiliaries have been shown to be useful in the acetate aldol reaction. The use of the parent Evans auxiliary with acetate substrates shows no diastereoselectivity.
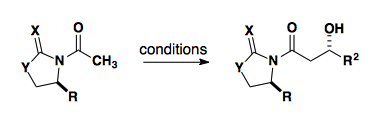
| X | Y | R | reference |
| S | S | t-Bu | Org. Lett., 2004, 6, 23-25. |
| S | S | C(CH3)2OTES | Org. Lett., 2004, 6, 3139-3141. |
| S | S or O | mesityl | Org. Lett., 2007, 9, 149-152. |
Mukaiyama Aldol
Enol silanes can be generated with high levels of enolate selectivity.
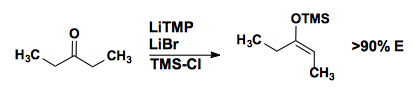
In the presence of a lewis acid, these enol silanes will add to aldehydes.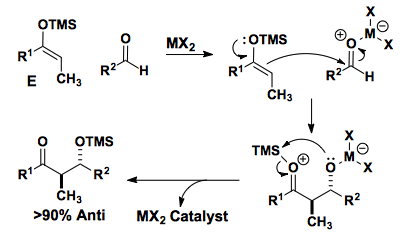
The Z-enol silane also reacts, albeit with similar anti-selectivity.
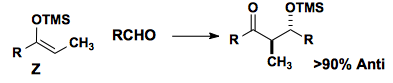
The Mukaiyama aldol reaction proceeds through an open transition state, which can be represented in a newman projection. The two illustrated stransition states do not differ significantly in energy, but there is a prevalent view that the anti-periplanar transition state is favored based on electrostatic effects.

Studies from the Heathcock group cast doubt on an inherent prefence.
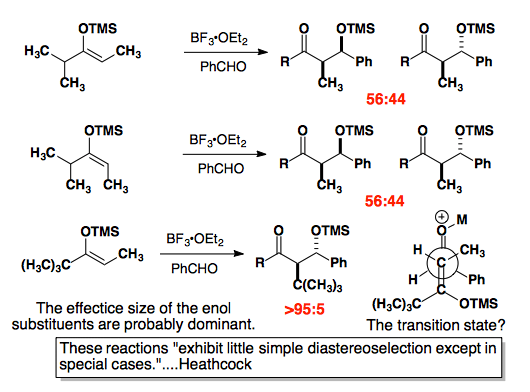
J. Org. Chem. 1986, 51, 3027-3037.
Asymmetric Catalysis
Despite the low inherent levels of diastereoselectivity in the Mukaiyama aldol reaction, several asymmetric, catalytic systems have been developed which rely on the catalyst to provide high levels of diastereoselectivity as well as enantioselectivity. The transition states of these reactions are believed to proceed through a closed transition state.
One of the major problems associated with catalyzing the Mukaiyama aldol is the possible generation of TMSOTf through the course of the reaction. This Lewis acid can then act as an achiral catalyst and erode the enantioselectivity of the reaction.
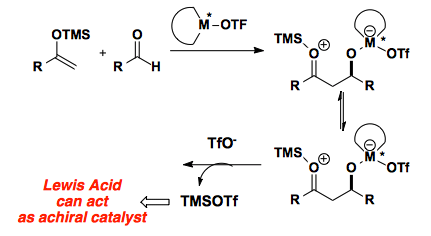
Additionally, Lewis acid catalyzed processes suffer from product inhibition of the active catalyst.
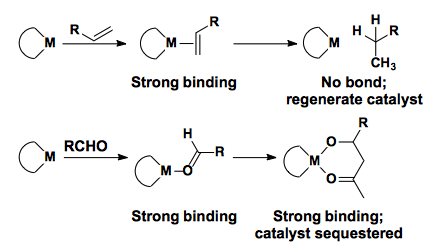
Yashimoto developed boron-based lewis acid catalysts that are readily available from tartrate or amino acids. These have been shown to convert the E- or Z-enolate to the anti-product in high diastereoselectivity and enantioselectivity.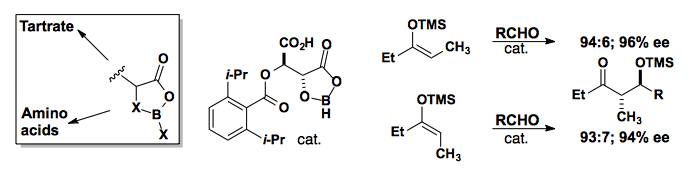
J. Am. Chem. Soc., 1991, 113, 1041-1042.
The Carreira group developed a titanium based lewis acid that allows for enantioselective acetate Mukaiyama aldol reactions.
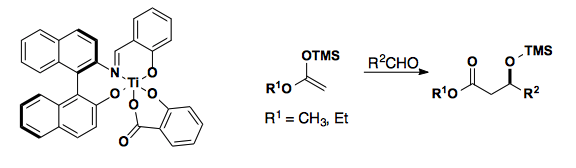
Aldol Alternatives
Access to the aldol retron (a beta-hydroxy carbonyl) can also be achieved by the Noyori reduction which will reduce a beta-keto ester to a beta-hydroxy ester.

