2.1: Atoms: Their Composition and Structure
- Page ID
- 50426
Learning Objectives
- Describe the structure of the atom
- Summarize the evolution of atomic theory based on landmark experiments by various scientists
- Define the properties of the subatomic particles including mass and charge
The Nuclear Atom- Overview
The precise physical nature of atoms finally emerged from a series of elegant experiments carried out between 1895 and 1915. The most notable of these achievements was Ernest Rutherford's famous 1911 alpha-ray scattering experiment, which established that
- Almost all of the mass of an atom is contained within a tiny (and therefore extremely dense) nucleus which carries a positive electric charge whose value identifies each element and is known as the atomic number of the element.
- Almost all of the volume of an atom consists of empty space in which electrons, the fundamental carriers of negative electric charge, reside. The extremely small mass of the electron (1/1840 the mass of the hydrogen nucleus) causes it to behave as a quantum particle, which means that its location at any moment cannot be specified; the best we can do is describe its behavior in terms of the probability of its manifesting itself at any point in space. It is common (but somewhat misleading) to describe the volume of space in which the electrons of an atom have a significant probability of being found as the electron cloud. The latter has no definite outer boundary, so neither does the atom. The radius of an atom must be defined arbitrarily, such as the boundary in which the electron can be found with 95% probability. Atomic radii are typically 30-300 pm.
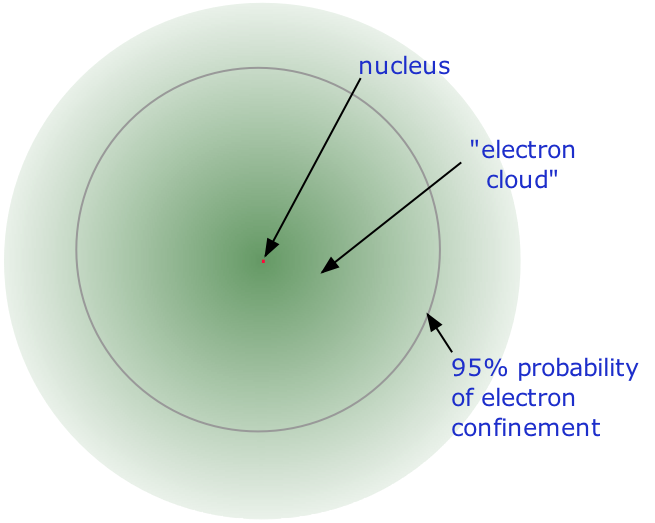
The nucleus is itself composed of two kinds of particles. Protons are the carriers of positive electric charge in the nucleus; the proton charge is exactly the same as the electron charge, but of opposite sign. This means that in any [electrically neutral] atom, the number of protons in the nucleus (often referred to as the nuclear charge) is balanced by the same number of electrons outside the nucleus. The other nuclear particle is the neutron. As its name implies, this particle carries no electrical charge. Its mass is almost the same as that of the proton. Most nuclei contain roughly equal numbers of neutrons and protons, so we can say that these two particles together account for almost all the mass of the atom.
Note
Because the electrons of an atom are in contact with the outside world, it is possible for one or more electrons to be lost, or some new ones to be added. The resulting electrically-charged atom is called an ion.
Atomic Theory through the Nineteenth Century
Early Greek Atomistic Philosophers
The earliest recorded discussion of the basic structure of matter comes from ancient Greek philosophers, the scientists of their day. In the fifth century BC, Leucippus and Democritus argued that all matter was composed of small, finite particles that they called atomos, a term derived from the Greek word for “indivisible.” They thought of atoms as moving particles that differed in shape and size, and which could join together. Later, Aristotle and others came to the conclusion that matter consisted of various combinations of the four “elements”—fire, earth, air, and water—and could be infinitely divided. Interestingly, these philosophers thought about atoms and “elements” as philosophical concepts, in contrast to the modern scientific method.
John Dalton's Atomic Theory
The Aristotelian view of the composition of matter held sway for over two thousand years, until English schoolteacher John Dalton helped to revolutionize chemistry with his hypothesis that the behavior of matter could be explained using an atomic theory. First published in 1807, many of Dalton’s hypotheses about the microscopic features of matter are still valid in modern atomic theory.
Five Postulates of Dalton's Atomic Theory
- Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change.
- An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element A macroscopic sample of an element contains an incredibly large number of atoms, all of which have identical chemical properties.
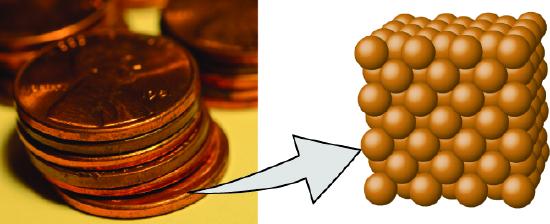
Figure \(\PageIndex{2}\): A pre-1982 copper penny (left) contains approximately \(3 \times 10^{22}\) copper atoms (several dozen are represented as brown spheres at the right), each of which has the same chemical properties. (credit: modification of work by “slgckgc”/Flickr)
- Atoms of one element differ in properties from atoms of all other elements.
- A compound consists of atoms of two or more elements combined in a small, whole-number ratio. In a given compound, the numbers of atoms of each of its elements are always present in the same ratio .
- Atoms are neither created nor destroyed during a chemical change, but are instead rearranged to yield substances that are different from those present before the change.
Atomic Theory late Nineteenth Century and Beyond
Discovery of the Electron
J.J. Thomson: Cathode Ray Tube
If matter is composed of atoms, what are atoms composed of? Are they the smallest particles, or is there something smaller? In the late 1800s, a number of scientists interested in questions like these investigated the electrical discharges that could be produced in low-pressure gases, with one of the most significant discoveries being made by English physicist J. J. Thomson using a cathode ray tube. This apparatus consisted of a sealed glass tube from which almost all the air had been removed; the tube contained two metal electrodes. When high voltage was applied across the electrodes, a visible beam called a cathode ray appeared between them. This beam was deflected toward the positive charge and away from the negative charge, and was produced in the same way with identical properties when different metals were used for the electrodes. In similar experiments, the ray was simultaneously deflected by an applied magnetic field, and measurements of the extent of deflection and the magnetic field strength allowed Thomson to calculate the charge-to-mass ratio of the cathode ray particles. The results of these measurements indicated that these particles were much lighter than atoms .
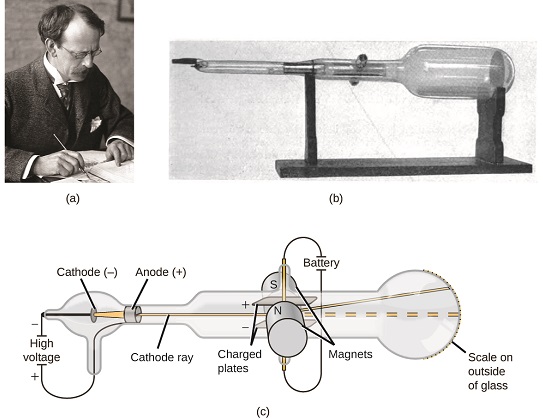
Based on his observations, here is what Thomson proposed and why: The particles are attracted by positive (+) charges and repelled by negative (−) charges, so they must be negatively charged (like charges repel and unlike charges attract); they are less massive than atoms and indistinguishable, regardless of the source material, so they must be fundamental, subatomic constituents of all atoms. Although controversial at the time, Thomson’s idea was gradually accepted, and his cathode ray particle is what we now call an electron, a negatively charged, subatomic particle with a mass less than a thousandth that of a proton. The term “electron” was coined in 1891 by Irish physicist George Stoney, from “electric ion.”
Robert Millikan Oil Drop Experiment
In 1909, more information about the electron was uncovered by American physicist Robert A. Millikan via his “oil drop” experiments. Millikan created microscopic oil droplets, which could be electrically charged by friction as they formed or by using X-rays. These droplets initially fell due to gravity, but their downward progress could be slowed or even reversed by an electric field lower in the apparatus. By adjusting the electric field strength and making careful measurements and appropriate calculations, Millikan was able to determine the charge on individual drops.
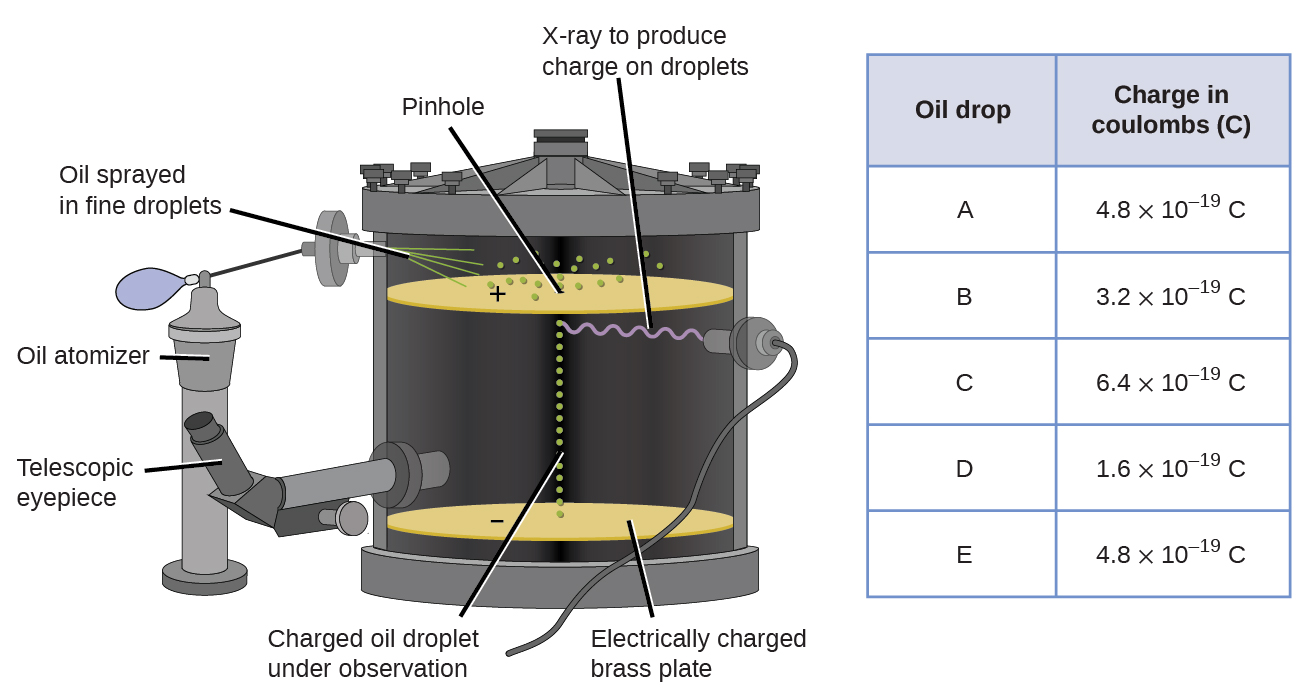
Looking at the charge data that Millikan gathered, you may have recognized that the charge of an oil droplet is always a multiple of a specific charge, 1.6 \(\times\) 10−19 C. Millikan concluded that this value must therefore be a fundamental charge—the charge of a single electron—with his measured charges due to an excess of one electron (1 times 1.6 \(\times\) 10−19 C), two electrons (2 times 1.6 \(\times\) 10−19 C), three electrons (3 times 1.6 \(\times\) 10−19 C), and so on, on a given oil droplet. Since the charge of an electron was now known due to Millikan’s research, and the charge-to-mass ratio was already known due to Thomson’s research (1.759 \(\times\) 1011 C/kg), it only required a simple calculation to determine the mass of the electron as well.
\[\mathrm{Mass\: of\: electron=1.602\times 10^{-19}\:\cancel{C}\times \dfrac{1\: kg}{1.759\times 10^{11}\:\cancel{C}}=9.107\times 10^{-31}\:kg} \tag{2.3.1}\]
Scientists had now established that the atom was not indivisible as Dalton had believed, and due to the work of Thomson, Millikan, and others, the charge and mass of the negative, subatomic particles—the electrons—were known. However, the positively charged part of an atom was not yet well understood. In 1904, Thomson proposed the “plum pudding” model of atoms, which described a positively charged mass with an equal amount of negative charge in the form of electrons embedded in it, since all atoms are electrically neutral. A competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturn-like atom, consisting of a positively charged sphere surrounded by a halo of electrons.
Figure \(\PageIndex{5}\): (a) Thomson suggested that atoms resembled plum pudding, an English dessert consisting of moist cake with embedded raisins (“plums”). (b) Nagaoka proposed that atoms resembled the planet Saturn, with a ring of electrons surrounding a positive “planet.” (credit a: modification of work by “Man vyi”/Wikimedia Commons; credit b: modification of work by “NASA”/Wikimedia Commons).
Radioactivity
The second line of investigation began in 1896, when the French physicist Henri Becquerel (1852–1908) discovered that certain minerals, such as uranium salts, emitted a new form of energy. Becquerel’s work was greatly extended by Marie Curie (1867–1934) and her husband, Pierre (1854–1906); all three shared the Nobel Prize in Physics in 1903. Marie Curie coined the term radioactivity (from the Latin radius, meaning “ray”) to describe the emission of energy rays by matter. She found that one particular uranium ore, pitchblende, was substantially more radioactive than most, which suggested that it contained one or more highly radioactive impurities. Starting with several tons of pitchblende, the Curies isolated two new radioactive elements after months of work: polonium, which was named for Marie’s native Poland, and radium, which was named for its intense radioactivity. Pierre Curie carried a vial of radium in his coat pocket to demonstrate its greenish glow, a habit that caused him to become ill from radiation poisoning well before he was run over by a horse-drawn wagon and killed instantly in 1906. Marie Curie, in turn, died of what was almost certainly radiation poisoning.

Building on the Curies’ work, the British physicist Ernest Rutherford (1871–1937) performed decisive experiments that led to the modern view of the structure of the atom. While working in Thomson’s laboratory shortly after Thomson discovered the electron, Rutherford showed that compounds of uranium and other elements emitted at least two distinct types of radiation. One was readily absorbed by matter and seemed to consist of particles that had a positive charge and were massive compared to electrons. Because it was the first kind of radiation to be discovered, Rutherford called these substances α particles. Rutherford also showed that the particles in the second type of radiation, β particles, had the same charge and mass-to-charge ratio as Thomson’s electrons; they are now known to be high-speed electrons. A third type of radiation, γ rays, was discovered somewhat later and found to be similar to a lower-energy form of radiation called x-rays, now used to produce images of bones and teeth.
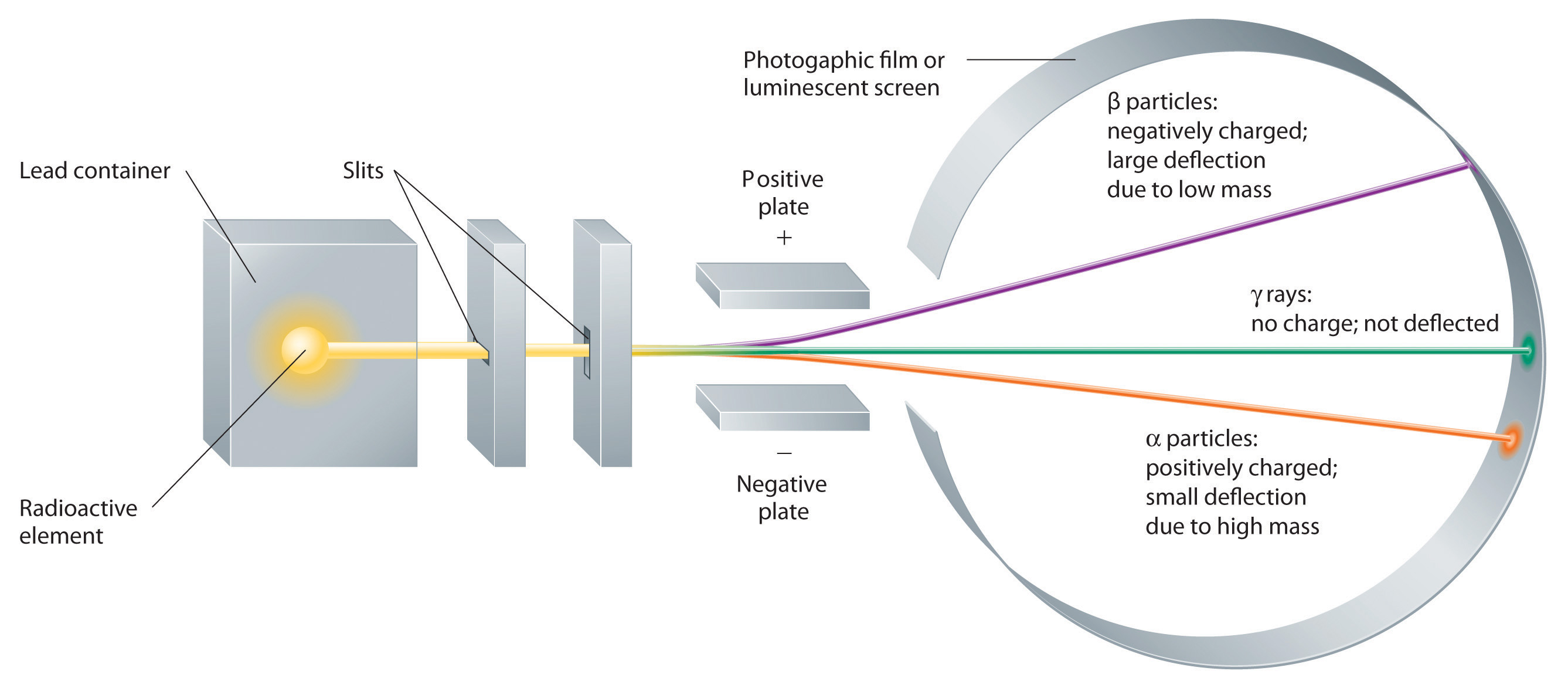
These three kinds of radiation—α particles, β particles, and γ rays—are readily distinguished by the way they are deflected by an electric field and by the degree to which they penetrate matter. As the Figure above illustrates, α particles and β particles are deflected in opposite directions; α particles are deflected to a much lesser extent because of their higher mass-to-charge ratio. In contrast, γ rays have no charge, so they are not deflected by electric or magnetic fields.
Three Common Types of Radiation
- Alpha (\(\alpha\))- helium nuclei (first to be discovered by Rutherford), are [+] and attracted towards negative electrodes. Under an equivalent electric field \(\alpha\) particles are deflected more than \(\beta\) particles and thus have a larger mass..
- Beta (\(\beta\)) - electrons (second to be discovered), are [-] and attracted towards positive electrodes. Under an equivalent electric field \(\beta\) particles are deflected less than \(\alpha\) particles and thus have a smaller mass..
- Gamma (\(\gamma\)) - high energy light waves (photons), neutral and not attracted towards electrodes.
Rutherford's Gold Foil Experiment and the atomic atom
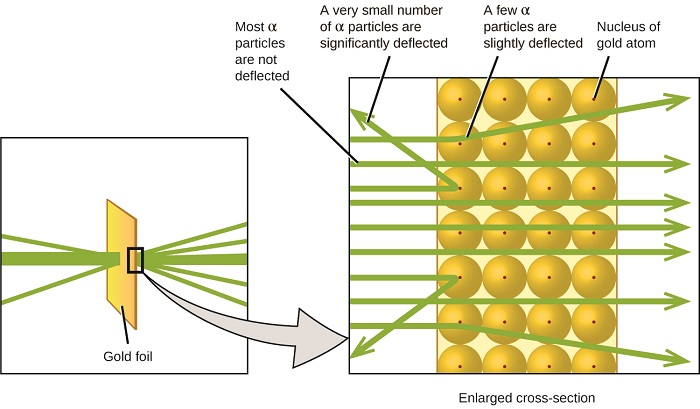
The next major development in understanding the atom came from Ernest Rutherford, a physicist from New Zealand who largely spent his scientific career in Canada and England. He performed a series of experiments using a beam of high-speed, positively charged alpha particles (α particles) that were produced by the radioactive decay of radium; α particles consist of two protons and two neutrons (you will learn more about radioactive decay in the Chapter on nuclear chemistry). Rutherford and his colleagues Hans Geiger (later famous for the Geiger counter) and Ernest Marsden aimed a beam of α particles, the source of which was embedded in a lead block to absorb most of the radiation, at a very thin piece of gold foil and examined the resultant scattering of the α particles using a luminescent screen that glowed briefly where hit by an α particle. What did they discover? Most particles passed right through the foil without being deflected at all. However, some were diverted slightly, and a very small number were deflected almost straight back toward the source (Figure \(\PageIndex{4}\)). Rutherford described finding these results: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you." (Nobel Laureates in Chemistry, 1901-1992, James Laylin, p. 57 ).
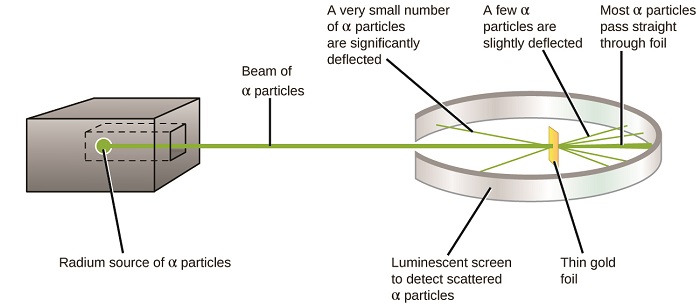
The following Phet simulation allows you to investigate Rutherford's gold foil experiment. At a minimum, you should run this simulatioin in both modes (Rutherford Atom and Plum Pudding), and understand how this experiment changed the scientific view of the atom, and led to the modern atomic model where most of the mass of matter is in the nucleus (neutrons and protons), and most of the volume is the void where the electron orbitals reside.
Here is what Rutherford deduced: Because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge (like charges repel each other). Since like charges repel one another, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly concentrated, positive charge. Since the deflections occurred a small fraction of the time, this charge only occupied a small amount of the space in the gold foil. Analyzing a series of such experiments in detail, Rutherford drew two conclusions:
- The volume occupied by an atom must consist of a large amount of empty space.
- A small, relatively heavy, positively charged body, the nucleus, must be at the center of each atom.
This analysis led Rutherford to propose a model in which an atom consists of a very small, positively charged nucleus, in which most of the mass of the atom is concentrated, surrounded by the negatively charged electrons, so that the atom is electrically neutral . After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a “building block,” and he named this more fundamental particle the proton, the positively charged, subatomic particle found in the nucleus. With one addition, which you will learn next, this nuclear model of the atom, proposed over a century ago, is still used today.
The Elements
To date, 118 different elements have been discovered; by definition, each is chemically unique. To understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components. Atoms consist of electrons, protons, and neutrons. Although this is an oversimplification that ignores the other subatomic particles that have been discovered, it is sufficient for discussion of chemical principles. Some properties of these subatomic particles are summarized in Table 2.1.1, which illustrates three important points:
- Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton, respectively.
- Neutrons have approximately the same mass as protons but no charge. They are electrically neutral.
- The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms.
The discovery of the electron and the proton was crucial to the development of the modern model of the atom and provides an excellent case study in the application of the scientific method. In fact, the elucidation of the atom’s structure is one of the greatest detective stories in the history of science.
| Particle | Mass (g)* | Atomic Mass (amu)* | Electrical Charge (coulombs) | Relative Charge |
|---|---|---|---|---|
| electron | 9.1093837015×10−28 | 0.0005485799090 | −1.602176634 × 10−19 | −1 |
| proton | 1.67262192369 ×10−24 | 1.007276466621 | +1.602 × 10−19 | +1 |
| neutron | 1.67492749804×10−24 | 1.00866491595 | 0 | 0 |
In most cases, the symbols for the elements are derived directly from each element’s name, such as C for carbon, U for uranium, Ca for calcium, and Po for polonium. Elements have also been named for their properties [such as radium (Ra) for its radioactivity], for the native country of the scientist(s) who discovered them [polonium (Po) for Poland], for eminent scientists [curium (Cm) for the Curie], for gods and goddesses [selenium (Se) for the Greek goddess of the moon, Selene], and for other poetic or historical reasons. Some of the symbols used for elements that have been known since antiquity are derived from historical names that are no longer in use; only the symbols remain to indicate their origin. Examples are Fe for iron, from the Latin ferrum; Na for sodium, from the Latin natrium; and W for tungsten, from the German wolfram. Examples are in Table 2.1.2.
| Element | Symbol | Derivation | Meaning |
|---|---|---|---|
| antimony | Sb | stibium | Latin for “mark” |
| copper | Cu | cuprum | from Cyprium, Latin name for the island of Cyprus, the major source of copper ore in the Roman Empire |
| gold | Au | aurum | Latin for “gold” |
| iron | Fe | ferrum | Latin for “iron” |
| lead | Pb | plumbum | Latin for “heavy” |
| mercury | Hg | hydrargyrum | Latin for “liquid silver” |
| potassium | K | kalium | from the Arabic al-qili, “alkali” |
| silver | Ag | argentum | Latin for “silver” |
| sodium | Na | natrium | Latin for “sodium” |
| tin | Sn | stannum | Latin for “tin” |
| tungsten | W | wolfram | German for “wolf stone” because it interfered with the smelting of tin and was thought to devour the tin |
Vocabulary
Test Yourself
Query \(\PageIndex{1}\)
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Elena Lisitsyna (H5P interactive modules)
- Anonymous
- Material adapted from Open Stax


