1A.4: Elements
- Page ID
- 50454
Learning Objectives
- Identify the name of the element given the symbol and vice versa
- Differentiate between atoms, elements, and molecules
Introduction
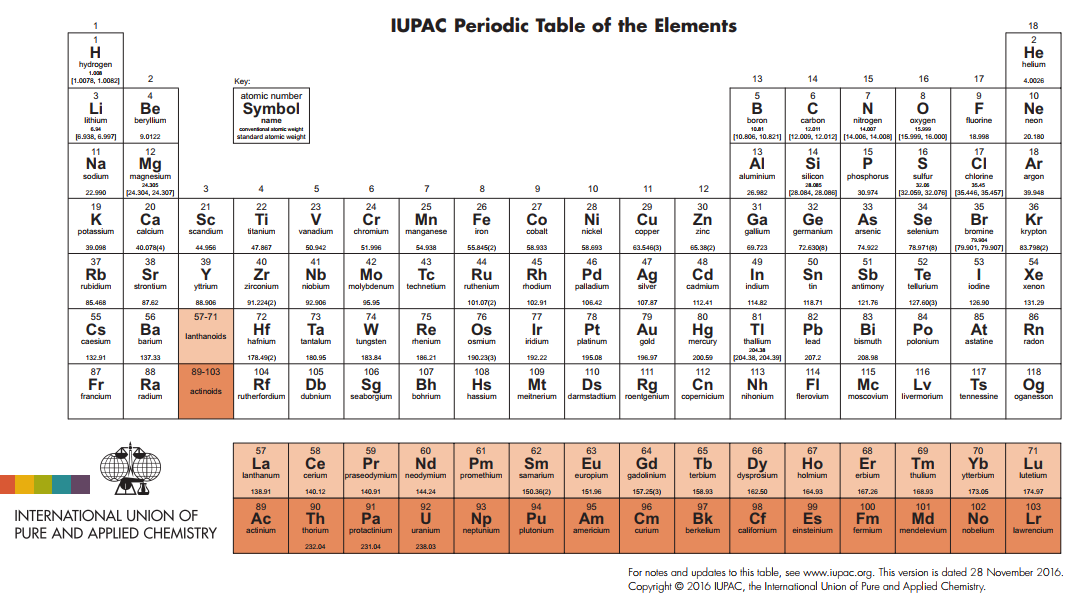
Atoms of the various elements can be considered to be the fundamental chemical entity of matter. There are 118 confirmed elements that can be arranged in the periodic table in a manner that allows chemists to predict behavior. Below is an official periodic table of the International Union of Pure and Applied Chemists (IUPAC). More information on the elements will be provided in Chapter 2.
Who is Responsible for Naming the Elements?
IUPAC, the International Union of Pure and Applied Chemistry. Here is a December 30, 2015 Press Release on the "Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118." To give you a feel for the challenges involved, here is a quote from the above Press Release:
“A particular difficulty in establishing these new elements is that they decay into hitherto unknown isotopes of slightly lighter elements that also need to be unequivocally identified” commented JWP chair Professor Paul J. Karol “but in the future we hope to improve methods that can directly measure the atomic number, Z.”
Note, isotopes and atomic numbers will be covered in Chapter 2.
What is the Difference Between an Atom and an Element?
An atom is a fundamental entity of matter that has a nucleus with one or more protons and zero or more neutrons, and containing electrons in orbitals around the nucleus. An element is a type of atom that is defined by the number of protons in the nucleus (see Chapter 2). The Atomic number is the number of protons in an atom. So all atoms with 6 protons are atoms of the element carbon.
Can Different Atoms of the Same Element have Different Masses?
Sure, if they have different numbers of neutrons. We call those Isotopes. Hydrogen has three isotopes, with all nuclei containing 1 proton and 0, (protium), 1 (deuterium) or 2 (tritium) neutrons.
Why Aren't Protons, Neutrons and Electrons Considered the Fundamental Chemical Entity?
Of course they are more fundamental than atoms, but they do not predict the behavior of the substance. An atom has a distinct number of protons and neutrons in its nucleus, and a specific number of electrons in identified orbitals. The chemical behavior is mostly determined by how many electrons the atom has, and what types of orbitals they are in (see Chapter 7). In chemical reactions the atoms of each element are conserved, and this will used to balance chemical equations in Chapter 3.
Are Atoms of a Type of Element Always Conserved in a Reaction?
No, some radiative process change the number of protons, and so an atom of one element becomes an atom of another element. These will be called Nuclear reactions, and will be covered in Chapter 23. Several of these radioactive decay processes will also be discussed in Chapter 2. But in chemical reactions, we will say yes, the atoms of each type of element are conserved.
UALR students in my class are required to know name and symbol of all the elements in this periodic table that are not shaded.
Click here for a Downloadable PDF
Test Yourself
The following Flash Cards require you to type the names of 22 elements that are commonly misspelled.
Query \(\PageIndex{1}\)
For additional practice on names you can do the following quiz.
Query \(\PageIndex{1}\)
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Ronia Kattoum (Learning Objectives)
- Elena Lisitsyna and Vincent Belford (H5P interactive modules)


