17.10 Reactions of Phenols
- Page ID
- 44260
Objectives
After completing this section, you should be able to
- explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see Section 16.4 of the textbook).
- write an equation to illustrate the oxidation of a phenol or an arylamine to a quinone, and identify the reagents used to oxidize phenols.
- write an equation to illustrate the reduction of a quinone to a hydroquinone, and identify the reagents used to reduce quinones.
- describe, briefly, the biological importance of the redox properties of quinones.
Key Terms
- hydroquinone (see the “Study Notes,” below)
- quinone (see the “Study Notes,” below)
- ubiquinone (see the “Study Notes,” below)
Study Notes
To assist you in learning the reactions of phenols, McMurry has divided the reactions into two groups: electrophilic aromatic substitution reactions (Objective 1), and oxidation reactions (Objectives 2-4)). “Quinone” is a term used to describe cyclohexadiendiones in general, and p-benzoquinone in particular. In addition to benzene, other aromatic systems also give rise to quinones; for example, 1,4-naphthoquinone
“Hydroquinones” are produced by the reduction of quinones according to the following half-reaction:
“Ubiquinones” are naturally occurring quinones whose role is to transfer a pair of electrons from one substance to another in enzyme-catalyzed reactions. Ubiquinones are also called coenzymes Q.
Electrophilic Aromatic Substitution Reactions
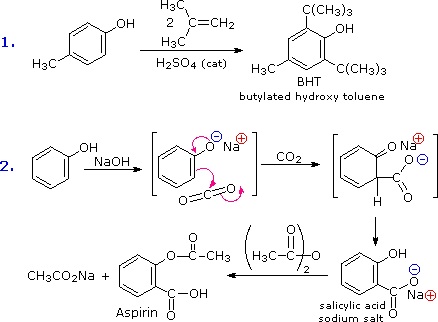
The facility with which the aromatic ring of phenols and phenol ethers undergoes electrophilic substitution has been noted. Two examples are shown in the following diagram. The first shows the Friedel-Crafts synthesis of the food preservative BHT from para-cresol. The second reaction is interesting in that it further demonstrates the delocalization of charge that occurs in the phenolate anion. Carbon dioxide is a weak electrophile and normally does not react with aromatic compounds; however, the negative charge concentration on the phenolate ring enables the carboxylation reaction shown in the second step. The sodium salt of salicylic acid is the major product, and the preference for ortho substitution may reflect the influence of the sodium cation. This is called the Kolbe-Schmidt reaction, and it has served in the preparation of aspirin, as the last step illustrates.
Oxidation of Phenols: Quinones
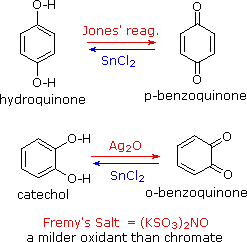
Phenols are rather easily oxidized despite the absence of a hydrogen atom on the hydroxyl bearing carbon. Among the colored products from the oxidation of phenol by chromic acid is the dicarbonyl compound para-benzoquinone (also known as 1,4-benzoquinone or simply quinone); an ortho isomer is also known. These compounds are easily reduced to their dihydroxybenzene analogs, and it is from these compounds that quinones are best prepared. Note that meta-quinones having similar structures do not exist. The redox equilibria between the dihydroxybenzenes hydroquinone and catechol and their quinone oxidation states are so facile that milder oxidants than chromate (Jones reagent) are generally preferred.
One such oxidant is Fremy's salt, shown on the right. Reducing agents other than stannous chloride (e.g. NaBH4) may be used for the reverse reaction. The position of the quinone-hydroquinone redox equilibrium is proportional to the square of the hydrogen ion concentration, as shown by the following half-reactions (electrons are colored blue). The electrode potential for this interconversion may therefore be used to measure the pH of solutions.
| Quinone + 2H(+) | 2e(–) –2e(–) | Hydroquinone |
|---|
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

