17.9 Phenols and Their Uses
- Page ID
- 44259
Objectives
After completing this section, you should be able to
- describe two methods that can be used to obtain phenol on an industrial scale.
- show how previously studied reactions are employed in the conversion of simple phenols into commercially significant compounds, such as BHT and 2,4-D.
Study Notes
You will not be examined on the mechanism shown in Figure 17.10 on page 651 of the textbook. One of the substances that has become symbolic of the struggle between industrial development and environmental protection is dioxin. The name dioxin is used to refer to a family of compounds having a basic structure in which two benzene rings are joined by two oxygen atoms:
However, media references to dioxin are usually in connection with 2,3,7,8-tetrachlorodibenzo-p-dioxin, or TCDD:
TCDD is a by-product formed in the manufacture of trichlorophenol, an intermediate in the production of the herbicide 2,4,5-T (one of the ingredients of the infamous Agent Orange), in the manufacture of hexachlorophene, by pulp and paper mills that use chlorine to produce white paper, and as a result of the incineration of municipal refuse.
The equation given below shows how 2,3,7,8-tetrachlorodibenzo-p-dioxin is formed during the manufacture of 2,4,5-trichlorophenol, an intermediate produced in the synthesis of 2,4,5-T. The process involves the reaction of 1,2,4,5-tetrachlorobenzenewith base at a temperature of about 160 ◦C.
The possible hazards presented by dioxin first became apparent in 1957, when some German workers involved in the manufacture of 2,4,5-T developed chloracne—a skin condition resembling acne. The precise toxicological effects of dioxin on humans is open to debate, but as little as 0.6 mg per kg of body weight will kill 50% of guinea pigs injected with TCDD within a specified time.
Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol. Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
An operation in 1753, painted by Gaspare Traversi, of a surgery before antiseptics were used.
One safer phenolic antiseptic is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene; resorcinol is the common name for 1,3-dihydroxybenzene, and 4-hexylresorcinol has a hexyl group on the fourth carbon atom of the resorcinol ring). It is much more powerful than phenol as a germicide and has fewer undesirable side effects. Indeed, it is safe enough to be used as the active ingredient in some mouthwashes and throat lozenges.
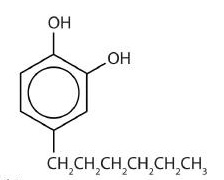
The compound 4-hexylresorcinol is mild enough to be used as the active ingredient in antiseptic preparations for use on the skin.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

