7.1 Industrial Preparation and Use of Alkenes
- Page ID
- 44296
Objectives
After completing this section, you should be able to
- discuss the industrial importance of ethylene (ethene) and propylene (propene).
- describe, briefly, the industrial process known as thermal cracking.
Study Notes
Table 7.1, below, identifies the industrial uses of the chemicals listed in Figure 7.1 on page 223 of Organic Chemistry, 8th ed.
Chemical Uses :
- ethanol solvent
- constituent of cleaning preparations;
- synthesis of esters
- acetaldehyde slug killer, in the form of methaldehyde, (CH3CHO)4
- acetic acid vinyl acetate polymers; ethyl acetate solvent;
- cellulose acetate polymers
- ethylene oxide preparation of “cellosolves” (industrial solvents)
- ethylene glycol anti-freeze; production of Dacron R
- ethylene dichloride solvent; production of vinyl chloride (see below)
- vinyl chloride manufacture of poly (vinyl chloride)—PVC
- vinyl acetate manufacture of poly (vinyl acetate) used in paint emulsions,
- plywood adhesives and textiles
- polyethylene “plastic” bags; toys; packaging
- isopropyl alcohol rubbing alcohol; cosmetics;
- synthesis of acetone
- propylene oxide polyurethanes;
- polyesters
- cumene industrial preparation of phenol and acetone
- polypropylene molded articles (e.g., kitchenware);
- carpet fibers for indoor-outdoor carpets
Catalytic cracking to form ethylene
Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst. The source of the large hydrocarbon molecules is often the naphtha fraction or the gas oil fraction from the fractional distillation of crude oil (petroleum). These fractions are obtained from the distillation process as liquids, but are re-vaporized before cracking.
The hydrocarbons are mixed with a very fine catalyst powder. These days the catalysts are zeolites (complex aluminosilicates) - these are more efficient than the older mixtures of aluminium oxide and silicon dioxide.
The whole mixture is blown rather like a liquid through a reaction chamber at a temperature of about 500°C. Because the mixture behaves like a liquid, this is known as fluid catalytic cracking (or fluidised catalytic cracking). Although the mixture of gas and fine solid behaves as a liquid, this is nevertheless an example of heterogeneous catalysis - the catalyst is in a different phase from the reactants.
The catalyst is recovered afterwards, and the cracked mixture is separated by cooling and further fractional distillation.
There isn't any single unique reaction happening in the cracker. The hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds. One possible reaction involving the hydrocarbon C15H32 might be:
![]()
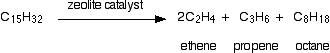
Or, showing more clearly what happens to the various atoms and bonds:
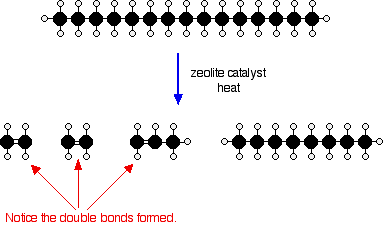
This is only one way in which this particular molecule might break up. The ethene and propene are important materials for making plastics or producing other organic chemicals. The octane is one of the molecules found in petrol (gasoline).
Ethene
Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst. The source of the large hydrocarbon molecules is often the naphtha fraction or the gas oil fraction from the fractional distillation of crude oil (petroleum). These fractions are obtained from the distillation process as liquids, but are re-vaporized before cracking.
There is not any single unique reaction happening in the cracker. The hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds. One possible reaction involving the hydrocarbon C15H32 might be:
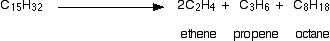
Or, showing more clearly what happens to the various atoms and bonds:
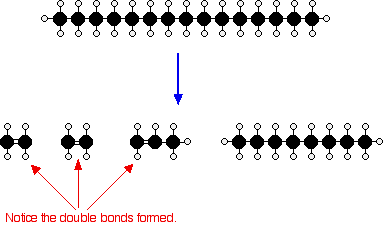
This is only one way in which this particular molecule might break up. The ethene and propene are important materials for making plastics or producing other organic chemicals. You will remember that during the polymeriation of ethene, thousands of ethene molecules join together to make poly(ethene) - commonly called polythene. The reaction is done at high pressures in the presence of a trace of oxygen as an initiator.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)

