18.9 Spectroscopy of Ethers
- Page ID
- 44289
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Objectives
After completing this section, you should be able to
- use the 1H NMR spectrum of an unknown ether or epoxide to determine its identity.
- identify the approximate chemical shift expected for protons attached to the carbon atoms that are bonded to oxygen in an ether or an epoxide.
Study Notes
You are not required to memorize the 13C NMR chemical shifts of ether carbon atoms.
Infrared Spectroscopy
Oxygen forms two bonds. An oxygen atom could be found in between two carbons, as in dibutyl ether.
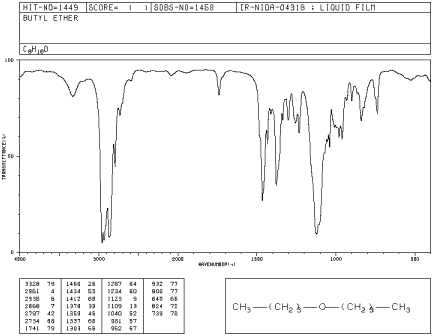
If you look at an IR spectrum of dibutyl ether, you will see:
- there are the usual sp3 C-H stretching and CH2 bending modes at 2900 and 1500 cm-1.
- there is a strong peak near 1000 cm-1. This peak is due to the C-O stretching vibration.
Figure IR7. IR spectrum of dibutyl ether. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
NMR Spectroscopy
- Hydrogens on carbon adjacent to the ether show up in the region of 3.4-4.5 ppm.
- Similar peaks in epoxides are shifted to a slightly higher field than other ethers. Hydrogens on carbons in and epoxide show up at 2.5 to 3.5 ppm.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)