11.1.5 Electrophilic Addition to Carbonyls
- Page ID
- 22810
It has been demonstrated that water, in the presence of an acid or a base, adds rapidly to the carbonyl function of aldehydes and ketones establishing a reversible equilibrium with a hydrate (geminal-diol or gem-diol). The word germinal or gem comes from the Latin word for twin, geminus.
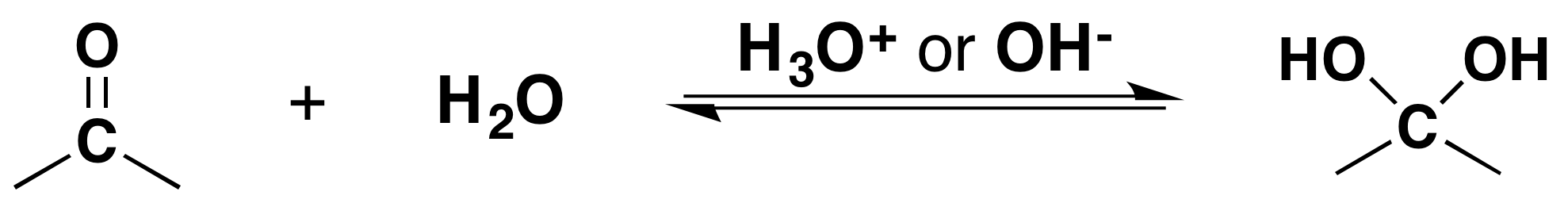
Going from Reactants to Products Simplified
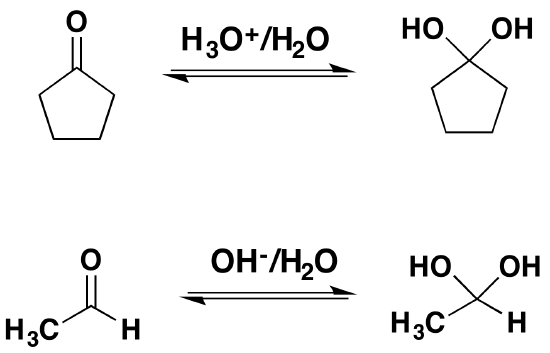
Reversibility of the Reaction
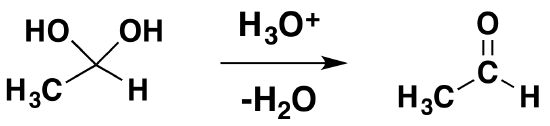
Isolation of gem-diols is difficult because the reaction is reversibly. Removal of the water during a reaction can cause the conversion of a gem-diol back to the corresponding carbonyl.
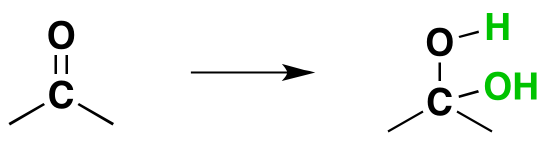
Factors Affecting the Gem-diol Equilibrium
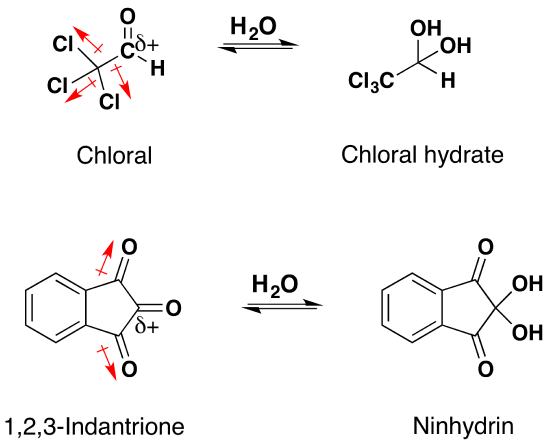
In most cases the resulting gem-diol is unstable relative to the reactants and cannot be isolated. Exceptions to this rule exist, one being formaldehyde where the weaker pi-component of the carbonyl double bond, relative to other aldehydes or ketones, and the small size of the hydrogen substituents favor addition. Thus, a solution of formaldehyde in water (formalin) is almost exclusively the hydrate, or polymers of the hydrate. The addition of electron donating alkyl groups stabilized the partial positive charge on the carbonyl carbon and decreases the amount of gem-diol product at equilibrium. Because of this ketones tend to form less than 1% of the hydrate at equilibrium. Likewise, the addition of strong electron-withdrawing groups destabilizes the carbonyl and tends to form stable gem-diols. Two examples of this are chloral, and 1,2,3-indantrione. It should be noted that chloral hydrate is a sedative and has been added to alcoholic beverages to make a “Knock-out” drink also called a Mickey Finn. Also, ninhydrin is commonly used by forensic investigators to resolve finger prints.
Mechanism of Gem-diol Formation
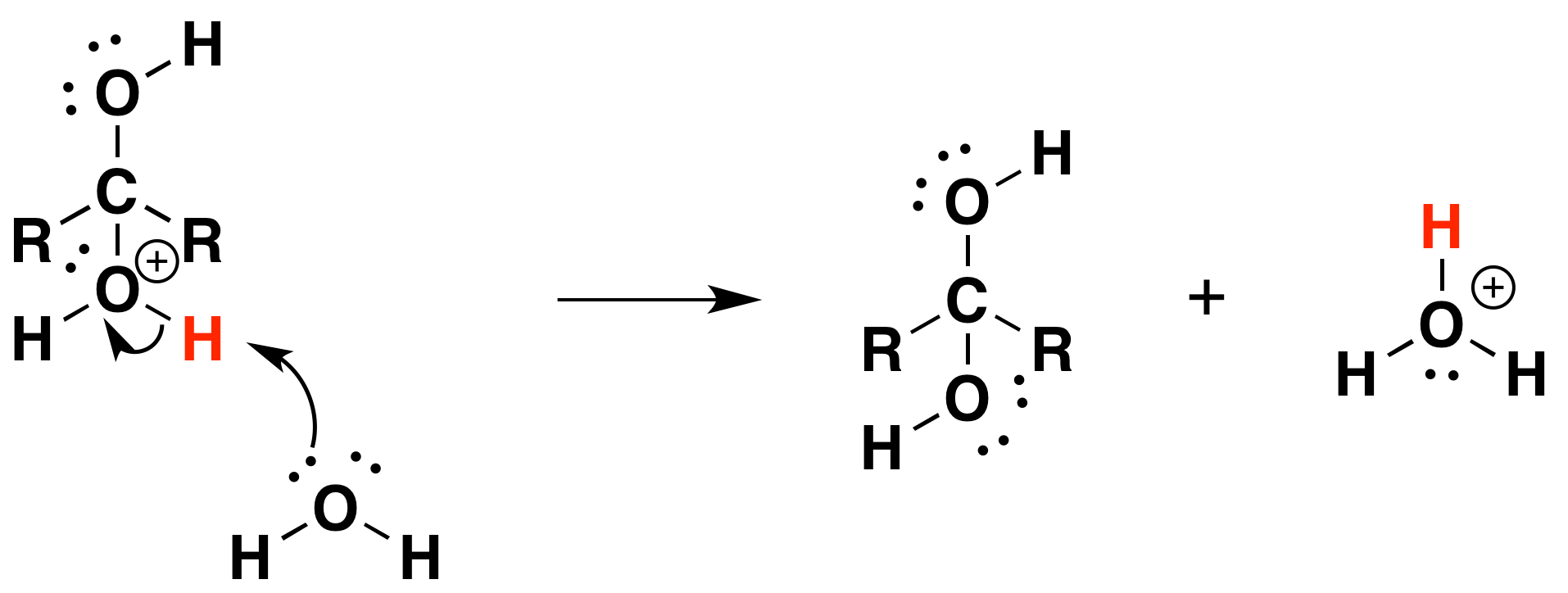
The mechanism is catalyzed by the addition of an acid or base. Note! This may speed up the reaction but is has not effect on the equilibriums discussed above. Basic conditions speed up the reaction because hydroxide is a better nucleophilic than water. Acidic conditions speed up the reaction because the protonated carbonyl is more electrophilic.
Under Basic conditions
1) Nucleophilic attack by hydroxide
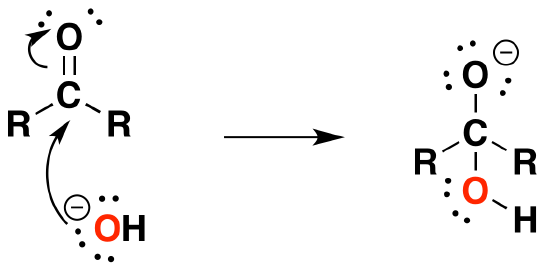
2) Protonation of the alkoxide

Under Acidic conditions
1) Protonation of the carbonyl
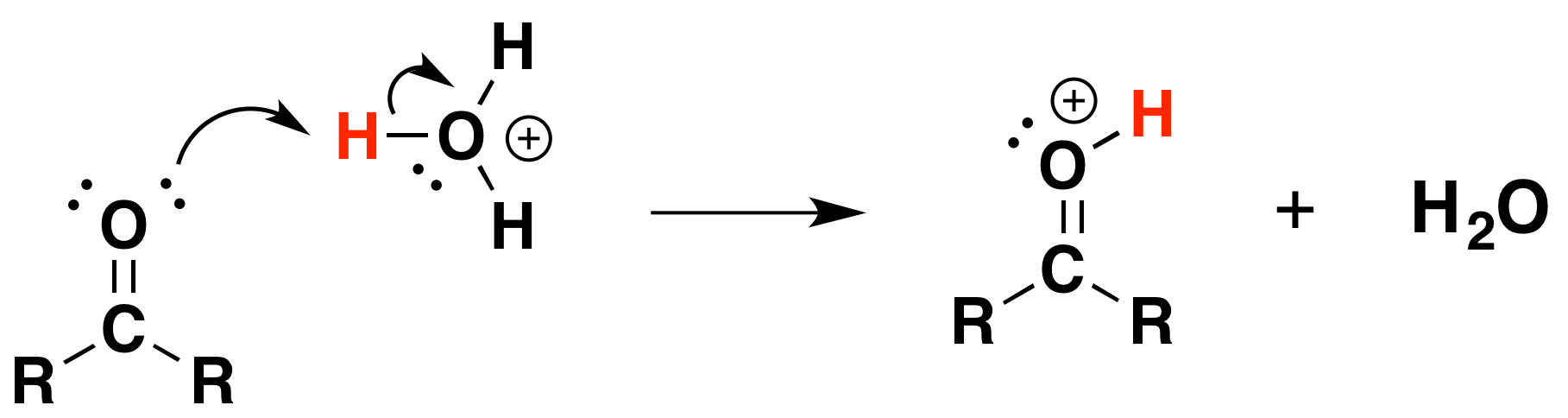
2) Nucleophilic attack by water
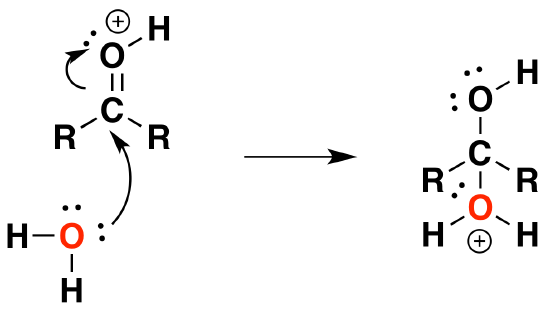
3) Deprotonation
Problems
1) Draw the expected products of the following reactions.

2) Of the following pairs of molecules which would you expect to form a larger percentage of gem-diol at equilibrium? Please explain your answer.

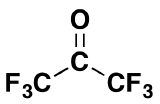
3) Would you expect the following molecule to form appreciable amount of gem-diol in water? Please explain your answer.
Answers
1)
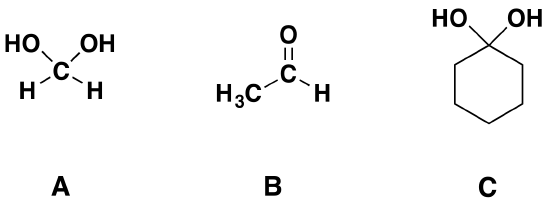
2) The compound on the left would. Fluorine is more electronegative than bromine and would remove more electron density from the carbonyl carbon. This would destabilize the carbonyl allowing for more gem-diol to form.
3) Although ketones tend to not form gem-diols this compound exists almost entirely in the gem-diol form when placed in water. Ketones tend to not form gem-diols because of the stabilizing effect of the electron donating alkyl group. However, in this case the electron donating effects of alkyl group is dominated by the presence of six highly electronegative fluorines.
Contributors
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
In this organic chemistry topic, we shall see how alcohols (R-OH) add to carbonyl groups. Carbonyl groups are characterized by a carbon-oxygen double bond. The two main functional groups that consist of this carbon-oxygen double bond are Aldehydes and Ketones.
Introduction
It has been demonstrated that water adds rapidly to the carbonyl function of aldehydes and ketones to form geminal-diol. In a similar reaction alcohols add reversibly to aldehydes and ketones to form hemiacetals (hemi, Greek, half). This reaction can continue by adding another alcohol to form an acetal. Hemiacetals and acetals are important functional groups because they appear in sugars.
To achieve effective hemiacetal or acetal formation, two additional features must be implemented. First, an acid catalyst must be used because alcohol is a weak nucleophile; and second, the water produced with the acetal must be removed from the reaction by a process such as a molecular sieves or a Dean-Stark trap. The latter is important, since acetal formation is reversible. Indeed, once pure hemiacetal or acetals are obtained they may be hydrolyzed back to their starting components by treatment with aqueous acid and an excess of water.
Formation of Hemiacetals

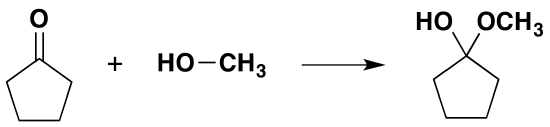
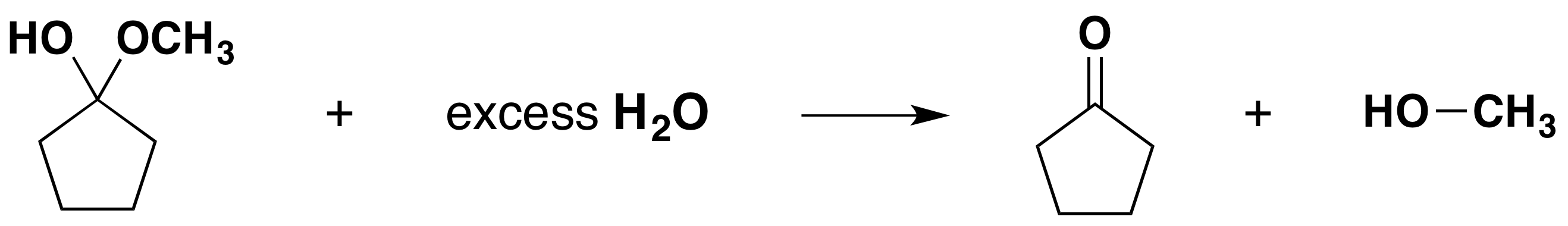
Formation of Acetals
Acetals are geminal-diether derivatives of aldehydes or ketones, formed by reaction with two equivalents (or an excess amount) of an alcohol and elimination of water. Ketone derivatives of this kind were once called ketals, but modern usage has dropped that term. It is important to note that a hemiacetal is formed as an intermediate during the formation of an acetal.

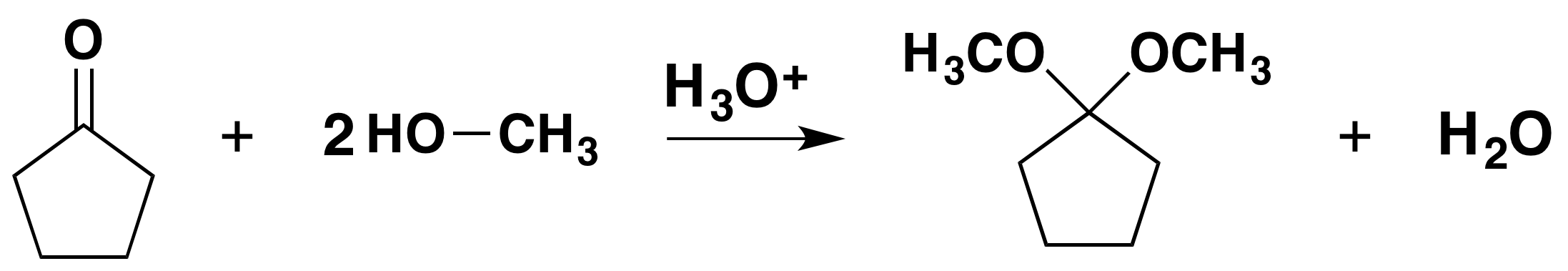
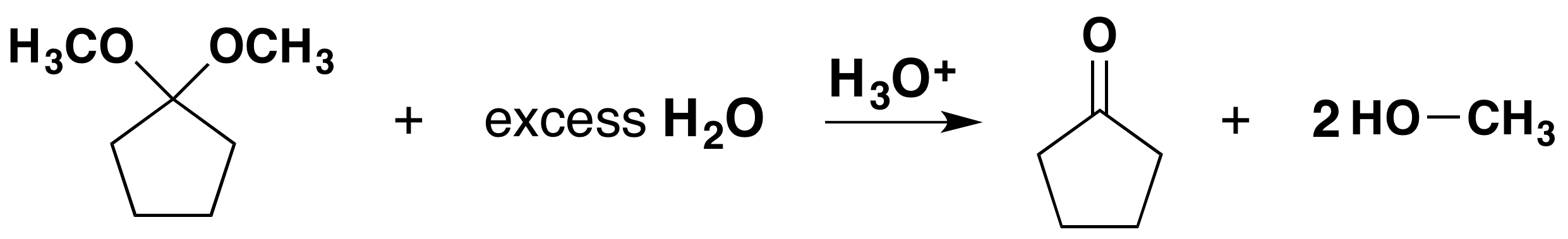
Mechanism for Hemiacetal and Acetal Formation
The mechanism shown here applies to both acetal and hemiacetal formation
1) Protonation of the carbonyl
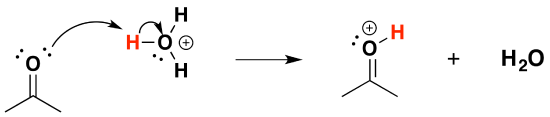
2) Nucleophilic attack by the alcohol
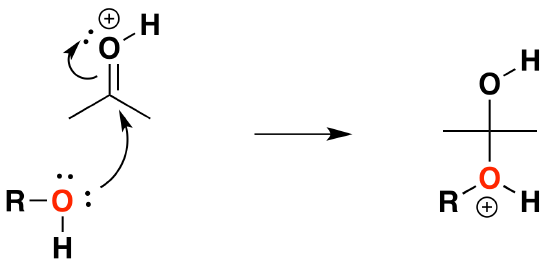
3) Deprotonation to form a hemiacetal

4) Protonation of the alcohol
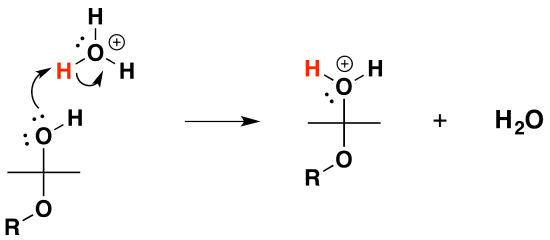
5) Removal of water
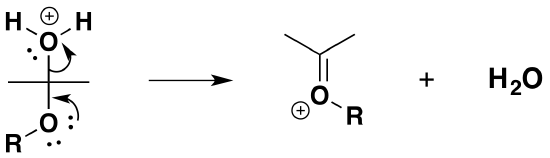
6) Nucleophilic attack by the alcohol
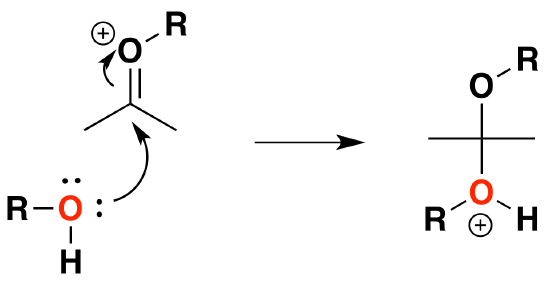
7) Deprotonation by water
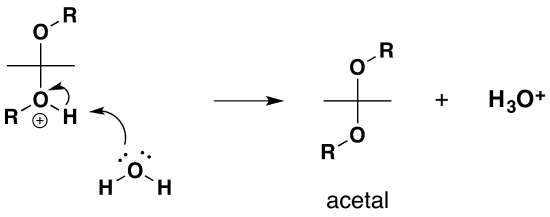
Formation of Cyclic Hemiacetal and Acetals
Molecules which have an alcohol and a carbonyl can undergo an intramolecular reaction to form a cyclic hemiacetal.
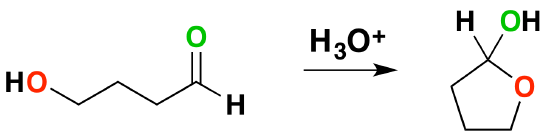
Intramolecular Hemiacetal formation is common in sugar chemistry. For example, the common sugar glucose exists in the cylcic manner more than 99% of the time in a mixture of aqueous solution.

Carbonyls reacting with diol produce a cyclic acetal. A common diol used to form cyclic acetals is ethylene glycol.

Acetals as Protecting Groups
The importance of acetals as carbonyl derivatives lies chiefly in their stability and lack of reactivity in neutral to strongly basic environments. As long as they are not treated by acids, especially aqueous acid, acetals exhibit all the lack of reactivity associated with ethers in general. Among the most useful and characteristic reactions of aldehydes and ketones is their reactivity toward strongly nucleophilic (and basic) metallo-hydride, alkyl and aryl reagents. If the carbonyl functional group is converted to an acetal these powerful reagents have no effect; thus, acetals are excellent protective groups, when these irreversible addition reactions must be prevented.
In the following example we would like a Grignard reagent to react with the ester and not the ketone. This cannot be done without a protecting group because Grignard reagents react with esters and ketones.
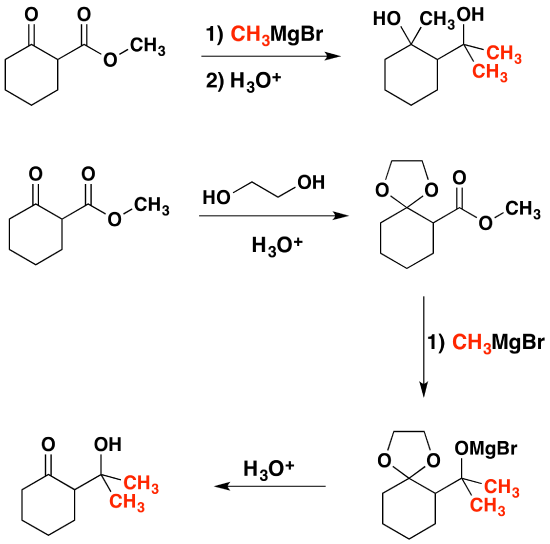
References
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. New York: W.H. Freeman and Company, 2007
- Carey, Francis. Advanced Organic Chemistry. 5th ed. Springer, 2007.
Contributors
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Ekram Alexander and Ahmed Rahim (UCD)

