Types and Nomenclature of Amines
- Page ID
- 26237
Amine bases are classified according to the number of alkyl or aryl groups attached to nitrogen. This number is important in determining the chemical reactions that are possible at the nitrogen atom:

A further classification exists if the nitrogen is multiply bonded to carbon, as in imines and aromatic nitrogen compounds:
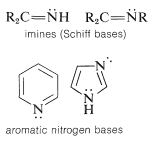
The nomenclature of amines was considered briefly in Section 7-8. We shall give only a short review here to focus on the main points. Amino compounds can be named either as derivatives of ammonia or as amino-substituted compounds:

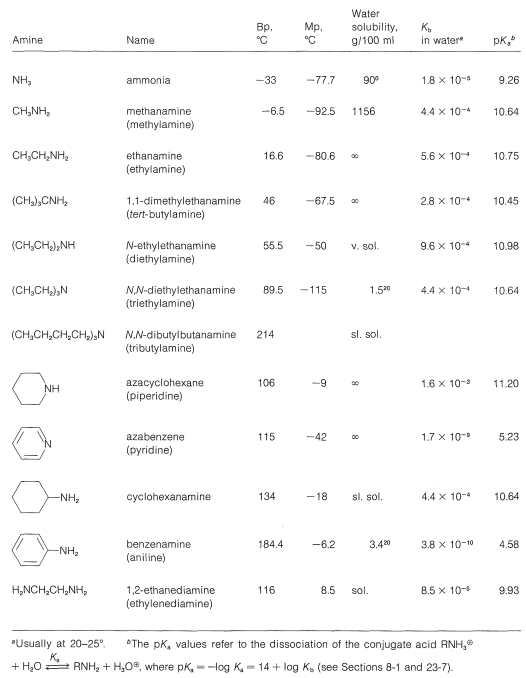
To be consistent and logical in naming amines as substituted ammonias, they strictly should be called alkanamines and arenamines, according to the nature of the hydrocarbon grouping. Unfortunately, the term alkylamine is used very commonly in place of alkanamine, while a host of trivial names are used for arenamines. We shall try to indicate both the trivial and the systematic names where possible. Some typical amines, their names, and their physical properties are listed in Table 23-1. The completely systematic names give in Table 23-1 illustrate in a poignant way the difficulty one gets into by using completely systematic names, and why simpler but less systematic names continue to be used for common compounds. A good example is \(\ce{N}\),\(\ce{N}\)-dibutylbutamine versus tributylamine. The special ways of naming heterocyclic amines are mentioned previously (Section 15-11A).
Table 23-1: Typical Amines and Their Properties
Salts of amines with inorganic or organic acids are named as substituted ammonium salts, except when the nitrogen is part of a ring system. Examples are
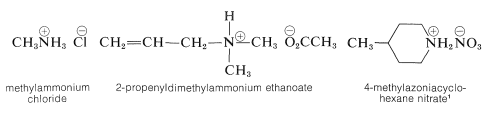
\(^1\)Note the use of azonia to denote the cationic nitrogen in the ring, whereas aza is used for neutral nitrogen (see Section 15-11A).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."

