Experiment 13: pH and its Relationship to Acids and Bases
- Page ID
- 98829
Learning Objectives:
- To determine the approximate pH of a variety of household solutions using red cabbage indicator.
- To accurately determine the pH of colored solutions using a pH meter.
- To test various solutions for the buffer behavior based on pH changes.
Introduction
Acids are defined as substances that dissolve in water producing hydrogen ions. The acidity of a solution is thus defined as its hydrogen ion concentration \([\ce{H}^+]\) expressed as the solution pH. The pH of a solution is more precisely defined as the negative log of the hydrogen ion concentration: \(pH = -\log[\ce{H}^+]\). The hydrogen ion in aqueous solution actually exists as the hydronium ion (\(\ce{H3O^{+}}\)) but it is simplified to \(\ce{H^{+}}\) in these expressions. Although there are no hard limits to the pH scale, the standard range extends from 0 to 14 with pH values below 7 classified as acidic and those above 7 as basic at room temperature.
Many plant substances contain richly colored pigment molecules that can be extracted from them using water. For example, red cabbage contains compounds that when extracted into water absorb wavelengths of light that cause the solution to appear purple. Interestingly, the compounds extracted from red cabbage leaves undergo structural changes in solution related to pH. These structural changes result in the compounds absorbing different wavelengths of light also depending on the solution pH. Correlated with the solution pH, it was observed that solutions of red cabbage leaf extracts also undergo a progression of distinctive color changes. These distinctive color changes have made it possible to use red cabbage leaf extracts to prepare a standard set of uniquely colored reference solutions that extend over the pH range of 1 to 13.
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
acidic neutral basic
Strong acids such as hydrochloric acid are substances that ionize completely in water producing hydronium ions. The ionization of hydrochloric acid is shown in Equations \ref{Eq1} and \ref{Eq2}. Equation is a further simplification of the first equation.
\[\begin{align} \ce{HCl (aq) + H2O(l) } & \ce{-> H3O+ (aq) + Cl- (aq) } \label{Eq1} \\[5pt] \ce{HCl (aq) } & \ce{-> H^{+}(aq) + Cl^{-} (aq) } \label{Eq2} \end{align}\]
Strong bases are substances that ionize completely in water producing hydroxide ions. The ionization of sodium hydroxide, a strong base, is shown as the simplified form in \ref{Eq3}. Sodium hydroxide on dissolving in water gives sodium ions and hydroxide ions.
\[\ce{NaOH (s) -> Na+ (aq) + OH- (aq) } \label{Eq3}\]
A similar logarithmic scale is also used to express the hydroxide ion concentration:
\[pOH = -\log[\ce{OH^{-}}].\]
There is an important inverse relationship between pH and pOH in that pOH values greater than 7 define solutions that are acidic! This is the result of the sum: pH + pOH = 14. Using this expression, one can determine the pOH if the pH is known. A solution at pH 5.5 would thus have a pOH of 8.5.
- Weak acids such as acetic acid (vinegar) and weak bases such as ammonia only ionize partially in water giving a much lower concentration of \(\ce{H+}\) and \(\ce{OH^{-}}\) ions.
- Buffers are substances that in aqueous solution resist changes in pH upon the addition of small amounts of an acid or small amounts of a base. This is accomplished because buffers contain both acidic and basic components. These two components in buffering substances don’t act to neutralize each other but are available to neutralize hydrogen ions or hydroxide ions from other sources.
Human blood is an important example of a buffered solution. The pH of blood is maintained between 7.35 and 7.45 by the bicarbonate system. Blood contains carbonic acid (\(\ce{H2CO3}\)), a weak acid, and its weak conjugate base, the bicarbonate anion (\(\ce{HCO3^{-}}\)). When excess acid or base enters the blood stream the conjugate acid/base system resists changes to the pH as shown in the equations below.
\[\begin{align} \underbrace{\ce{H2CO3 (aq)}}_{\text{Weak acid}} + \ce{OH^{-}(aq)} &\ce{-> } \underbrace{\ce{HCO3^{-}(aq)}}_{\text{Weak base}} + \ce{H2O(l) } \label{Eq4a} \\[5pt] \underbrace{\ce{HCO3^{-}(aq)}}_{\text{Weak base}} + \ce{ H^{+}(aq)} &\ce{ -> } \underbrace{\ce{H2CO3 (aq) }}_{\text{Weak acid}} \label{Eq5a} \end{align}\]
Calculations
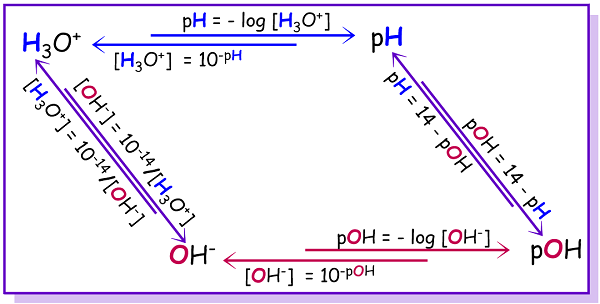
Scheme 1: Flow Chart for Calculating [H+], [OH-], pH and pOH
a. The hydrogen ion and hydroxide ion concentrations are inversely proportional in aqueous solutions. Knowing the concentration of either one makes it possible to calculate the other using Equation \ref{Eq5}.
\[K_w = [\ce{H^{+}}][\ce{OH^{-}}] = 1.0 \times 10^{-14} \label{Eq5}\]
The \(K_w\) value of \(1.0 \times 10^{-14}\) only strictly holds true for solutions at 25°C
b. pH and pOH of an aqueous solution can be calculated using the expressions:
\[pH = -\log[\ce{H^{+}}] \label{Eq6}\]
\[pOH = -\log[\ce{OH^{-}}] \label{Eq7}\]
c. For a given pH of an aqueous solution, the concentration of hydrogen ions is calculated using:
\[[\ce{H^{+}}] = 10^{-pH} \label{Eq8}\]
d. The relationship between pH and pOH at 25 °C:
\[ pH + pOH = 14.00 = pK_w \label{Eq9}\]
Example \(\PageIndex{1}\):
Determine the hydrogen ion concentration, pH and pOH of a solution with a hydroxide ion concentration of \(1.8 \times 10^{-10} M\).
Solution
Use Equation \ref{Eq5}:
\[\begin{align*} [\ce{H^{+}}] [1.8 \times 10^{-10}] &= 1.0 \times 10^{-14} \\[5pt] [\ce{H^{+}}] &= \dfrac{1.0 \times 10^{-14}}{1.8 \times 10^{-10} M } \\[5pt] &= 5.6 \times 10^{-5} \end{align*}\]
Use Equation \ref{Eq6}:
\[\begin{align*} pH &= - \log[\ce{H^{+}}] \\[5pt] &= - \log [5.6 \times 10^{-5}] \\[5pt] &= 4.25 \end{align*}\]
The solution is acidic since \(pH<7\)
Use Equation \ref{Eq9}:
\[pH + pOH = 14.00\]
\[4.25 + pOH = 14.00 \]
thus \(pOH = 9.75\)
Example \(\PageIndex{2}\)
Determine the \([\ce{H^{+}}]\) and the \(\ce{[OH^{-}]}\) of a solution whose pH is 3.5.
Solution
Use Equation \ref{Eq8}:
\[[H^+] = 10^{-pH} = 10^{-3.5} = 3.2 \times 10^{-4} M\]
Use Equation \ref{Eq5} and rearrange:
\[[H+][OH-] = 1.0 \times 10^{-14} \nonumber \]
\[\begin{align*} [\ce{OH^{-}}] &= \dfrac{ 1.0 \times 10^{-14}}{3.2 \times 10^{-4} } \\[5pt] &= 3.1 \times 10^{-11}\,M \end{align*}\]
Experimental Procedure
Part 1: Preparing a pH Reference Set
You will be using a purple extract derived from red cabbage leaves as an indicator to prepare a reference set of standard solutions ranging in pH values from 1 to 13.
Students at each laboratory bench will prepare a standard reference pH set. Buffer solutions ranging in pH from 1 to 13 will be provided.
- Label 13 test tubes as 1-13.
- Measure 4 mL of the pH 1 buffer in test tube #1
- Use tube 1 as a reference to gauge the 4 mL volume
- Transfer 4 mL of the remaining buffers to the other tubes.
- Use a plastic transfer pipette to add 1 mL of the cabbage leaf indicator to each of the tubes.
- Swirl the test tubes sideways to thoroughly mix the contents.
This will produce thirteen uniquely colored solutions that will be used as references to determine the pH of different samples. Arrange the colored solutions in the test tube rack and record the color that corresponds to each pH in Table I. Be creative and use unique terms to describe the colors. For example, record cherry red instead of just red.
Part 2 pH Determination of Various Samples
A. Transfer 4 mL of a clear sample into a test tube and add 1 mL of the cabbage leaf indicator. Compare the color of the resulting solution to the reference set and record the color and the pH in Table IIA. Record the pH of the standard reference tube that most closely resembles the color of your sample. If the color of the sample appears in-between two of the reference tubes, record the lower value and add 0.5. Repeat this procedure to test all of the samples.
B. It is difficult and usually unreliable to use colored indicators for samples that are also colored because the color of the pigment will interfere with making observations of the indicator as its color changes. In colored samples the solution pH is more accurately determined using a pH meter.
Several different colored samples will be in small beakers located near the pH meters.
- Remove the pH electrode from the storage solution
- Rinse the electrode with distilled water.
- Gently blot dry the electrode using a Kimwipe.
- Carefully lower the pH electrode into the sample to be tested.
- Wait for the display to stabilize.
- The pH of the solution appears on the display.
- Record the pH value in Table II-B.
- Remove the electrode from the solution.
- Rinse the electrode with distilled water
- Blot the electrode dry with a Kimwipe.
- Repeat this procedure with the remaining solutions.
- Record the pH for each sample in Table II-B.
- Do a final rinse of the electrode with distilled water
- Blot the electrode dry with a Kimwipe.
- Return the electrode to the buffered storage solution.
- Check to be sure the electrode tip is immersed in the buffer solution.
Part 3 Testing for Buffer Behavior Based on pH Changes
A. Transfer 4 mL of the samples listed below into separate clean test tubes. Add 1 mL of the cabbage leaf indicator and determine the pH of each sample. Record the pH of each solution in Table III-A.
- 0.2 M NaCl
- Distilled water
- 0.2 M NaC2H3O2
- Solution A
- Solution B
Then add 5 drops of 0.1 M HCl solution to each of the above samples. Swirl and thoroughly mix the solution. It may be necessary to use a clean stirring rod to achieve complete mixing. Record the pH of these new solutions and calculate the change in pH units from the original samples. Record the absolute difference for each in the │ΔpH│ column of Table III-A.
B. Repeat the procedures from above with the same 5 solutions. Record the pH of the initial solutions in Table III- B. Then add 5 drops of 0.1 M NaOH this time instead of acid to each of the tubes and swirl to thoroughly mix the solutions. Record the pH of these new solutions and calculate the change in pH units from the initial values. Record the absolute differences for each in the │ΔpH│ column of Table III-B.
Clean Up and Waste Disposal
• Discard all the reagents down the drain with plenty of water.
• Wash the test tubes
• Rinse the test tubes with purified water.
• Place them open side down in the test tube rack.
• Return general equipment to its proper location in the lab.
• Clean the benchtop with a wet paper towel.

