Chapter 7.6: Chemical Reactions in the Atmosphere
- Page ID
- 18859
Learning Objectives
- To become familiar with the various reactions implicated in the destruction of Earth’s ozone layer.
Section 6.5 described different classes of chemical reactions. Of the many different chemical reactions that occur in Earth’s atmosphere, some are important and controversial because they affect our quality of life and health. The atmospheric reactions presented in this section provide examples of the various classes of reactions introduced in this chapter that are implicated in the destruction of Earth’s protective ozone layerA concentration of ozone in the stratosphere (about 1015 ozone molecules per liter) that acts as a protective screen, absorbing ultraviolet light that would otherwise reach the surface of the earth, where it would harm plants and animals..
 Each year since the mid-1970s, scientists have noted a disappearance of approximately 70% of the ozone (O3) layer above Antarctica during the Antarctic spring, creating what is commonly known as the “ozone hole.” OzoneAn unstable form of oxygen that consists of three oxygen atoms bonded together (O3). A layer of ozone in the stratosphere helps protect the plants and animals on earth from harmful ultraviolet radiation. Ozone is responsible for the pungent smell we associate with lightning discharges and electric motors. It is also toxic. is an unstable form of oxygen that consists of three oxygen atoms bonded together. In September 2009, the Antarctic ozone hole reached 24.1 million km2 (9.3 million mi2), about the size of North America. The largest area ever recorded was in the year 2000, when the hole measured 29.9 million km2 and for the first time extended over a populated area—the city of Punta Arenas, Chile (population 154,000; Figure 7.6.1). A less extensive zone of depletion has been detected over the Arctic as well in some years, particularly those in which the temperature of the stratosphere is very cold. Years of study from the ground, from the air, and from satellites in space have shown that chlorine from industrial chemicals used in spray cans, foam packaging, and refrigeration materials is largely responsible for the catalytic depletion of ozone through a series of condensation, cleavage, and oxidation–reduction reactions.
Each year since the mid-1970s, scientists have noted a disappearance of approximately 70% of the ozone (O3) layer above Antarctica during the Antarctic spring, creating what is commonly known as the “ozone hole.” OzoneAn unstable form of oxygen that consists of three oxygen atoms bonded together (O3). A layer of ozone in the stratosphere helps protect the plants and animals on earth from harmful ultraviolet radiation. Ozone is responsible for the pungent smell we associate with lightning discharges and electric motors. It is also toxic. is an unstable form of oxygen that consists of three oxygen atoms bonded together. In September 2009, the Antarctic ozone hole reached 24.1 million km2 (9.3 million mi2), about the size of North America. The largest area ever recorded was in the year 2000, when the hole measured 29.9 million km2 and for the first time extended over a populated area—the city of Punta Arenas, Chile (population 154,000; Figure 7.6.1). A less extensive zone of depletion has been detected over the Arctic as well in some years, particularly those in which the temperature of the stratosphere is very cold. Years of study from the ground, from the air, and from satellites in space have shown that chlorine from industrial chemicals used in spray cans, foam packaging, and refrigeration materials is largely responsible for the catalytic depletion of ozone through a series of condensation, cleavage, and oxidation–reduction reactions.
Figure 7.6.1 Satellite Photos of Earth Reveal the Sizes of the Antarctic Ozone Hole over Time
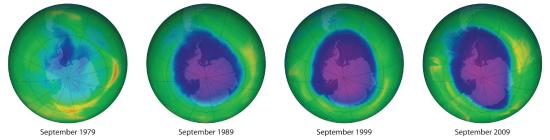
Dark blue colors correspond to the thinnest ozone; light blue, green, yellow, orange, and red indicate progressively thicker ozone. In September 2000, the Antarctic ozone hole briefly approached a record 30 million km2.
Earth’s Atmosphere and the Ozone Layer
The Earth’s atmosphere at sea level is an approximately 80:20 solution of nitrogen and oxygen gases, with small amounts of carbon dioxide, water vapor, and the noble gases, and trace amounts of a variety of other compounds (Table 7.6.1 ).
Table 7.6.1: The composition 9of the Earth's Atmosphere at Sea Level*
| Gas | Formula | Volume (%) |
|---|---|---|
| nitrogen | N2 | 78.084 |
| oxygen | O2 | 20.948 |
| argon | Ar | 0.934 |
| carbon dioxide† | CO2 | 0.0314 |
| neon | Ne | 0.00182 |
| helium | He | 0.000524 |
| krypton | Kr | 0.000114 |
| methane | CH4 | 0.0002 |
| hydrogen | H2 | 0.00005 |
| nitrous oxide | N2O | 0.00005 |
| xenon | Xe | 0.0000087 |
| * In addition, air contains as much as 7% water vapor (H2O), 0.0001% sulfur dioxide (SO2), 0.00007% ozone (O3), 0.000002% carbon monoxide (CO), and 0.000002% nitrogen dioxide (NO2). | ||
| † Carbon dioxide levels are highly variable at the surface because of biological photosynthesis and respiration and human combustion of fossil fuels; the typical range is 0.01–0.1%. Above a few hundred meters where local effects are washed out by the air circulation the concentration of carbon dioxide is consistant at 400 ppmV | ||
Carbon dioxide levels are highly variable near the surface due to combustion (our contribution) and photosynthesis and respiration of plants. Above a few hundred meters, where the atmosphere is well mixed, CO2 is consistent. In preindustrial times this was ~280 ppmV. Today it is 400 ppmV, an increase of over 30% There is a relatively small (~10 ppm) seasonal variation which is observed in the northern hemisphere due to the annual blooming of plants in the spring, but this is not seen in the southern hemisphere which has much more ocean.
A key feature of the atmosphere is that its temperature, and pressure vary dramatically with altitude. Consequently, scientists have divided the atmosphere into distinct layers, which interact differently with the continuous flux of solar radiation from the top and the land and ocean masses at the bottom. Some of the characteristic features of the layers of the atmosphere are illustrated in Figure 7.6.2
Figure 7.6.2 Variation of Temperature with Altitude in Earth’s Atmosphere
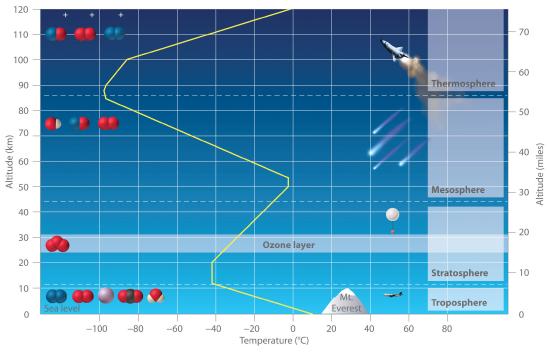
Note the important chemical species present in each layer. The yellow line indicates the temperature at various altitudes.
The troposphere is the lowest layer of the atmosphere, the troposphere extends from earth’s surface to an altitude of about 11–13 km (7–8 miles). The temperature of the troposphere decreases steadily with increasing altitude. is the lowest layer of the atmosphere, extending from Earth’s surface to an altitude of about 11–13 km (7–8 mi). Above the troposphere lies the stratosphere, which extends from 13 km (8 mi) to about 44 km (27 mi). As shown in Figure 7.6.2, the temperature of the troposphere decreases steadily with increasing altitude. Because “hot air rises,” this temperature gradient leads to continuous mixing of the upper and lower regions within the layer. The thermally induced turbulence in the troposphere produces fluctuations in temperature and precipitation that we collectively refer to as “weather.” The turbulance also means that the atmosphere is well mixed. The percentage composition except for water vapor and ozone is the same up to about 100 km altitude. Water vapor composition varies because water vapor is condensible, and at lower temperatures will condense out into water droplets. Thus the water vapor concentration falls off quickly with altitude. For example, there is almost no water vapor in the stratosphereIn contrast, mixing between the layers of the atmosphere occurs relatively slowly, so each layer has distinctive chemistry. We focus our attention on the stratosphere, which contains the highest concentration of ozone.The sun’s radiation is the major source of energy that initiates chemical reactions in the atmosphere. The sun emits many kinds of radiation, including visible lightRadiation that the human eye can detect., which is radiation that the human eye can detect, and ultraviolet lightHigh-energy radiation that cannot be detected by the human eye but can cause a wide variety of chemical reactions that are harmful to organisms., which is higher energy radiation that cannot be detected by the human eye. This higher energy ultraviolet light can cause a wide variety of chemical reactions that are harmful to organisms. For example, ultraviolet light is used to sterilize items, and, as anyone who has ever suffered a severe sunburn knows, it can produce extensive tissue damage.Light in the higher energy ultraviolet range is almost totally absorbed by oxygen molecules in the upper layers of the atmosphere, causing the O2 molecules to dissociate into two oxygen atoms in a cleavage reaction:
\( O_{2}\left ( g \right )\overset{light}{\rightarrow}2O\left ( g \right ) \tag{7.6.1} \)
In Equation 7.6.1, light is written above the arrow to indicate that light is required for the reaction to occur. The oxygen atoms produced in Equation 7.6.1 can undergo a condensation reaction with O2 molecules to form ozone:
\( O\left ( g \right ) + O_{2}\left ( g \right ) \rightarrow O_{3}\left ( g \right ) \tag{7.6.2} \)
Ozone is responsible for the pungent smell we associate with lightning discharges and electric motors. It is also toxic and a significant air pollutant, particularly in cities. In the stratosphere, the ozone produced via Equation 7.6.2 has a major beneficial effect. All of the UV light below 300 nm is absorbed either by oxygen molecules (below 200 nm) or ozone (between 200 and 300 nm) in the stratosphere. None reaches the surface. This is the mechanism by which the surface and living creatures on it are protected from damaging UV light. The source of ozone in the troposphere is not the photolysis of oxygen, but the emission of NO2(g) from cars. The NO2(g) can absorb light between 420 and 300nm to yield oxygen atomsOzone absorbs the less-energetic range of ultraviolet light, undergoing a cleavage reaction in the process to give O2 and O:
\( O_{3} \left ( g \right ) \overset{light}{\rightarrow} O_{2}\left ( g \right )+ O\left ( g \right ) \tag{7.6.3} \)
The formation of ozone (Equation 7.6.2) and its decomposition (Equation 7.6.3) are normally in balance, resulting in essentially constant levels of about 1015 ozone molecules per liter in the stratosphere. This so-called ozone layer acts as a protective screen that absorbs ultraviolet light that would otherwise reach Earth’s surface.In 1974, F. Sherwood Rowland and Mario Molina published a paper claiming that commonly used chlorofluorocarbon (CFC) compounds were causing major damage to the ozone layer (Table 7.6.2 ). CFCs had been used as refrigerants and propellants in aerosol cans for many years, releasing millions of tons of CFC molecules into the atmosphere. Because CFCs are volatile compounds that do not readily undergo chemical reactions, they persist in the atmosphere long enough to be carried to the top of the troposphere, and eventually enter the stratosphere. There they are exposed to intense ultraviolet light and undergo a cleavage reaction to produce a chlorine atom, which is shown for Freon-11:
\( CCl_{3}F \left ( g \right ) \overset{light}{\rightarrow} CCl_{2}F\left ( g \right )+ Cl\left ( g \right ) \tag{7.6.4} \)
The resulting chlorine atoms act as a homogeneous catalyst in two redox reactions (Equation 7.6.5 and Equation 7.6.6 ):
\( Cl\left ( g \right )+O_{3} \left ( g \right ) \rightarrow ClO \left ( g \right )+ O_{2} \left ( g \right ) \tag{7.6.5} \)
\( ClO \left ( g \right )+O \left ( g \right ) \rightarrow Cl \left ( g \right )+ O_{2} \left ( g \right ) \tag{7.6.6} \)
Adding the two reactions in Equation 7.6.5 and Equation 7.6.6 gives
\( Cl \left ( g \right )+O_{3} \left ( g \right )+ClO\left ( g \right )+O\left ( g \right ) \rightarrow ClO \left ( g \right )+ Cl\left ( g \right )+ 2O_{2} \left ( g \right ) \tag{7.6.7} \)
Because chlorine and ClO (chlorine monoxide) appear on both sides of the equation, they can be canceled to give the following net reaction:
\( O_{3} \left ( g \right )+O\left ( g \right ) \rightarrow 2O_{2} \left ( g \right ) \tag{7.6.8} \)
In the presence of chlorine atoms, one O3 molecule and one oxygen atom react to give two O2 molecules. Although chlorine is necessary for the overall reaction to occur, it does not appear in the net equation. The chlorine atoms are a catalyst that increases the rate at which ozone is converted to oxygen.
Table 7.6.2 Common CFCs and Related Compounds
| Name | Molecular Formula | Industrial Name |
|---|---|---|
| trichlorofluoromethane | CCl3F | CFC-11 (Freon-11) |
| dichlorodifluoromethane | CCl2F2 | CFC-12 (Freon-12) |
| chlorotrifluoromethane | CClF3 | CFC-13 (Freon-13) |
| bromotrifluoromethane | CBrF3 | Halon-1301* |
| bromochlorodifluoromethane | CBrClF2 | Halon-1211 |
| *Halons, compounds similar to CFCs that contain at least one bromine atom, are used as fire extinguishers in specific applications (e.g., the engine rooms of ships). | ||
Because the stratosphere is relatively isolated from the layers of the atmosphere above and below it, once chlorine-containing species enter the stratosphere, they remain there for long periods of time. Each chlorine atom produced from a CFC molecule can lead to the destruction of large numbers of ozone molecules, thereby decreasing the concentration of ozone in the stratosphere. Eventually, however, the chlorine atom reacts with a water molecule to form hydrochloric acid, which is carried back into the troposphere and then washed out of the atmosphere in rainfall.
The Ozone Hole
Massive ozone depletions were first observed in 1975 over the Antarctic at the end of polar winter when the atmosphere is again exposed to sunlight. A similar, although much more limited depletion has been observed in the Arctic during very cold winters
Although the reactions in Equation 7.6.6 and Equation 7.6.7 appear to account for most of the ozone destruction observed at low to middle latitudes it is not responsible for the ozone hole. It is a common misconception that the ozone hole is a result of ozone depletion during the winter. This is not the case. While ozone is not formed over the pole during the winter (see Equations 7.6.1 and 7.6.2), neither is it destroyed. As a matter of fact ozone actually increases over the South Pole during the winter due to transport from the mid-latitude stratosphere and the lack of destruction by sunlight (Equations 7.6.3). Formation of Cl atoms and ClO (Equation 7.6.4) also requires UV sunlight to generate chlorine atoms, and sunlight is in very short supply during the polar winters. However there is interesting chemistry going on in the stratosphere over the South Pole during the winter which sets the stage for the rapid springtime ozone depletion that leads to formation of the ozone hole
Research has shown that, chlorine monoxide reacts with stratospheric nitrogen dioxide in a redox reaction to form chlorine nitrate (ClONO2). Chlorine nitrate is a "storage" species for ClO. The ClO in ClONO2 is not available for reaction with oxygen atoms. When chlorine nitrate is in the presence of trace amounts of HCl or adsorbed on ice particles in stratospheric clouds, additional redox reactions can occur in which chlorine nitrate produces Cl2 or HOCl (hypochlorous acid):
\( HCl \left ( g \right )+ClONO_{2} \left ( g \right ) \rightarrow Cl_{2} \left ( g \right )+ HNO_{3}\left ( g \right ) \tag{7.6.9} \)
\( H_{2}O \left ( g \right )+ClONO_{2} \left ( g \right ) \rightarrow HOCl \left ( g \right )+ HNO_{3}\left ( g \right ) \tag{7.6.10} \)
Both Cl2 and HOCl undergo photodissociation reactions in even weak sunlight to yield reactive chlorine atoms. When the sun finally rises after the long polar night, relatively large amounts of Cl2 and HOCl are present and rapidly generate high levels of chlorine atoms. The reactions shown in Equation 7.6.5 and Equation 7.6.6 then cause ozone levels to fall dramatically.Stratospheric ozone levels decreased about 2.5% from 1978 to 1988, which coincided with a fivefold increase in the widespread use of CFCs since the 1950s. If the trend were allowed to continue, the results could be catastrophic. Fortunately, all countries have banned the use of CFCs in aerosols. In 1987, representatives from 43 nations signed the Montreal Protocol, committing themselves to reducing CFC emissions by 50% by the year 2000. Later, representatives from a large number of countries, alarmed by data showing the rapid depletion of stratospheric chlorine, agreed to phase out CFCs completely by the early 21st century; the United States banned their use in 1995. The projected effects of these agreements on atmospheric chlorine levels are shown in Figure 7.6.3. Because of the very slow rate at which CFCs are removed from the stratosphere, however, stratospheric chlorine levels will not fall to the level at which the Antarctic ozone hole was first observed until about 2050. The scientific community recognized Molina and Rowland’s work in 1995, when they shared the Nobel Prize in Chemistry.Figure
7.6.3 Projected Effects of International Agreements on Atmospheric Chlorine Levels
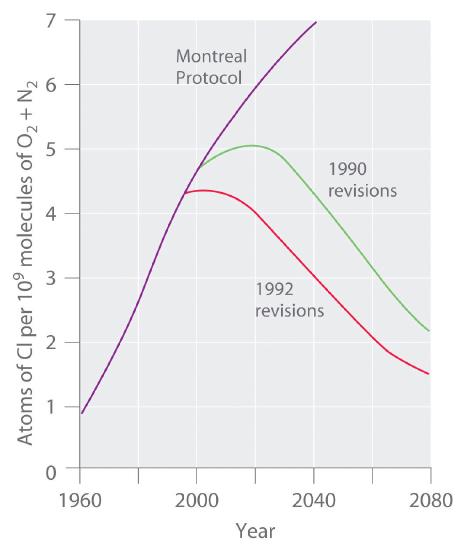
The graph plots atmospheric chlorine content in chlorine atoms per 109 molecules of O2 plus N2 from 1960 to 1990 (actual data) and 1990 to 2080 (estimated for various schemes for regulating CFC emissions).
Manufacturing companies have been successful in finding alternatives to the CFCs used in the air-conditioning units of cars, houses, and commercial buildings. One approach is to use hydrochlorofluorocarbons (HCFCs), hydrocarbons in which only some of the hydrogen atoms are replaced by chlorine or fluorine, and hydrofluorocarbons (HFCs), which do not contain chlorine (Table 7.6.3 ). The C–H bonds in HCFCs and HFCs act as “handles” that permit additional chemical reactions to occur in the troposphere. Consequently, these substances are degraded more rapidly, and most are washed out of the atmosphere before they can reach the stratosphere
Table 7.6.3 Selected HCFCs and HFCs
| Name | Molecular Formula | Industrial Name |
|---|---|---|
| chlorodifluoromethane | CHClF2 | HCFC-22 (freon-22) |
| 1-chloro-1,1-difluoroethane | CH3CClF2 | HCFC-141b |
| 2,2-dichloro-1,1,1-trifluoroethane | CHCl2CF3 | HCFC-123 |
| 1,1,1,2-tetrafluoroethane | CH2FCF3 | HFC-134a |
HFCs are used as a replacement for CFCs. The molecular structure of HFC-134a is shown in this ball-and-stick model.Nonetheless, the small fraction of HCFCs that reaches the stratosphere will deplete ozone levels just as CFCs do, so they are not the final answer. Indeed, the 1990 London amendment to the Montreal Protocol specifies that HCFCs must be phased out by 2040. Finding suitable replacements for refrigerants is a subject of ongoing research for chemists in the 21st century.It should be noted that political opponents of the Montreal Protocols told us that replacements could not be found, that developing countries such as India and China would never stop manufacturing CFCs and that the costs of replacements would tax the economy. They were wrong.
EXAMPLE 16
In the 1970s Hal Johnson at Cal Tech proposed that ozone could be catalytically destroyed by the nitric oxides, NO and NO2, however within a few years it was shown that this was not a serious threat, for among other reasons because increasing NOx concentrations in the stratosphere would favor production of additional ClONO2 further sequestering ClO. One source of NOx is combustion of hydrocarbons in engines and NOx from car engines is a key source of ozone pollution in the troposphere. Jets flying in the troposphere also produce NOx, but tropospheric NOx never reaches the stratosphere because of reactions with water leading to the formation of nitric acid, which rains out. We will discuss the role of nitric acid in acid rain later. The fact that high-flying supersonic aircraft would inject NO directly into the stratosphere was a major argument against the development of commercial supersonic transports and Johnson's identification of NOx catalytic destruction of ozone was one of the threads which lead Rowland and Molina to their studies of Cl atom reactions with ozone.
Given: identity of compound
Asked for: Predict what reactions are likely to occur between NO and ozone.
Solution: Both NO and NO2 are known oxides of nitrogen. Thus NO is likely to react with ozone according to the chemical equation
\( NO \left ( g \right )+O_{3} \left ( g \right ) \rightarrow NO_{2} \left ( g \right )+ O_{2}\left ( g \right ) \)
NO2(g) also reacts with atomic oxygen according to the equation
\( NO_{2} \left ( g \right )+O \left ( g \right ) \rightarrow NO \left ( g \right )+ O_{2}\left ( g \right ) \)
leading to a potential catalytic cycle for ozone destruction similar to that caused by chlorine atoms. Based on these reactions, the development of commercial supersonic transports was not recommended until the environmental impact had undergone additional testing. Rowland and Molina's work was a development of those studies
Exercise:
An industrial manufacturer proposed that halons such as CF3Br could be used as replacements for CFC propellants. Do you think that this is a reasonable suggestion or is there a potential problem with such a use?
 Answer: Because the compound CF3Br contains carbon, fluorine, and a bromine atom that is chemically similar to chlorine, it is likely that it would also be a catalyst for ozone destruction. There is therefore a potential problem with its use. As a matter of fact, halons (bromine containing fluorocarbons) have extremely high ozone destruction potentials because bromine atoms drive a much higher number of ozone catalytic destruction cycles before termination. Because halons have crucial applications including use as fire extinguishers in aircraft and other sectors, and because it has been very difficult to find replacements for them they have been very hard to replace and remain an issue of concern. Emissions of methyl bromide used in agriculture have also proved difficult to limit.
Answer: Because the compound CF3Br contains carbon, fluorine, and a bromine atom that is chemically similar to chlorine, it is likely that it would also be a catalyst for ozone destruction. There is therefore a potential problem with its use. As a matter of fact, halons (bromine containing fluorocarbons) have extremely high ozone destruction potentials because bromine atoms drive a much higher number of ozone catalytic destruction cycles before termination. Because halons have crucial applications including use as fire extinguishers in aircraft and other sectors, and because it has been very difficult to find replacements for them they have been very hard to replace and remain an issue of concern. Emissions of methyl bromide used in agriculture have also proved difficult to limit.Summary
Key Takeaway: The composition of Earth’s atmosphere is vulnerable to degradation through reactions with common industrial chemicals.
Conceptual Problems
Numerical Problem
Contributors
- Anonymous
- Modified by Joshua Halpern



