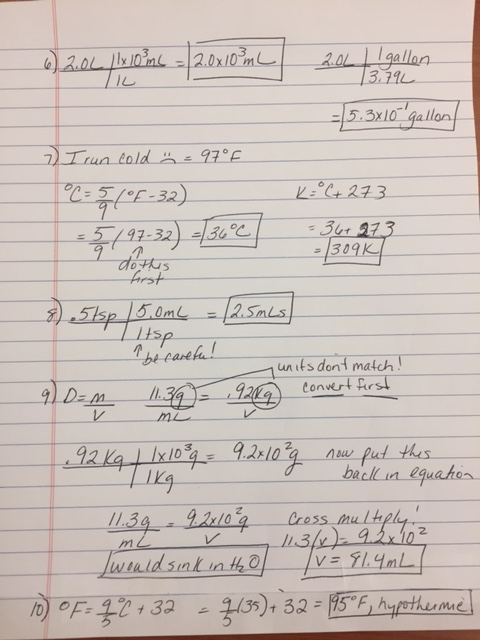2.E: The Mathematics of Chemistry (Exercises)
- Page ID
- 85142
End of Chapter Exercises
1) Convert 45.0 cm3 to L.
2) Acetone (CH3COCH3) has a density of .791g/mL. Assuming this compound is soluble in water, how many grams are present in 3.0 L of acetone. If acetone was spilled in Furman Lake, what would you expect to see and how would this be classified in the matter scheme (element,compound, heterogeneous, or homogeneous mixture?
3) Convert the distance from Greenville, South Carolina to Atlanta, Georgia. The measured length of the trip is approximately 235 kilometers. Convert this distance to meters and miles.
4) In January, the average temperature of eastern Siberia is -50°C. Calculate this temperature in Fahrenheit and Kelvin. Of the three temperature units, which one is the SI unit for temperature. (Kelvin temperature answer is incorrect on answer key should be 223.15K)
5) The mass of an adult human eyeball is 7.5 grams. Convert this value to kilograms and ounces. What type of lab equipment is used to measure mass?
6) How many milliliters and gallons are contained in a 2.0L Coca-Cola drink? (1 gallon = 3.79 L)
7) Convert your body temperature to °F and K.
8) Your cat needs .5 tsp of Tylenol. How many milliliters is this dosage?
9) The density of lead is 11.3g/mL. If you measured out .92 kg, how many milliliters would this mass displace? Based upon density only, would you expect lead to sink or float in water?
10) If you have a body temperature of 35°C, are you hypothermic or hyperthermic?
Solutions
* #4 should be 223K*