3.6: Stereoisomers
- Page ID
- 83490
Stereoisomers are isomers that have the same molecular formula and ligands, but differ in the arrangement of those ligands in 3D space.
Introduction
Isomers are molecules that have the same molecular formula but differ in the way the atoms are arranged around the central atom. For example, a molecule with the formula AB2C2, has two ways it can be drawn:
Isomer 1: Isomer 2:
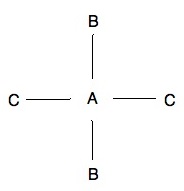
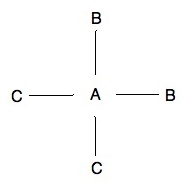
The above two pictures are examples of isomers, specifically cis-trans isomers which we will discuss below. One thing to remember whenever talking about stereoisomers is that all other options that may come to mind are just rotations of the existing isomers. For example, a molecule that has both C's on the top right plane and both B's on the bottom left plane is not another isomer. Instead it is just a 180o rotation of isomer 2.
Stereoisomers are isomers that mainly differ in the way the ligands or atoms are placed relative to the central atom. There are two types of stereoisomers:
- Geometric Isomers = Isomers that differ in the way the ligand is bound to the metal.
- Optical Isomers = Isomers that do not have symmetry and are not superimposable on their mirror images.
In terms of their differences, geometric isomers show much more activity than optical isomers. What this statement means is that optical isomers often display similar properties and do not seem all that different. That is until they react with other optical isomers or when they react with light. As discussed later on, one of the main ways optical isomers are detected is their ability to polarize or change the direction of light. Geometric isomers on the other hand exhibit different properties from one isomer to another.
Review of Molecular Geometry
To understand stereoisomers, one must understand all the possible molecular geometries. For stereoisomers, only these geometries will be relevant:
- Linear
- Square Planar
- Tetrahedral
- Octahedral
It is also important to remember the coordination number associated with these geometries. Coordination numbers refer to the number of ligands or atoms bound to the central atom. Thus, linear has a coordination number of 2 because it consists of 2 atoms bound to the central atom. Square planar and tetrahedral have a coordination number of 4 while octahedral has a coordination number of 6. These geometries are very important as they dictate whether or not certain isomers exist.
Linear
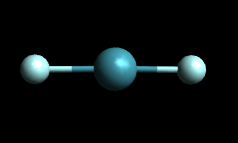
An example of linear geometry is provided below of the molecule Xenon Difluoride (XeF2). Recall that linear is the geometry where the molecule looks like a line.
XeF2:
Square Planar
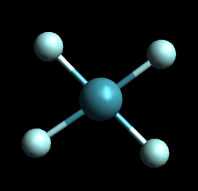
Square planar is the geometry where the molecule looks like it is a square plane. An example of the molecule Xenon Tetrafluoride (XeF4) is provided below.
XeF4:
Tetrahedral
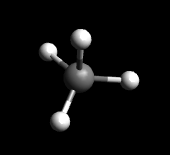
Tetrahedral is the geometry where the molecule looks like a pyramid. An example of the molecule Methane (CH4) is provided below.
CH4:
Octahedral
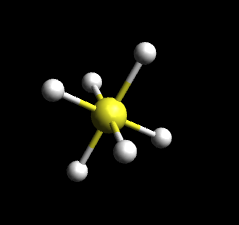
Octahedral is the geometry where the bases of two pyramids are stuck together. Alternatively, it can also be though as where the center consists of a square plane with a ligand sticking out above it and another ligand sticking out below it. An example of the molecule Sulfur Hexafluoride (SF6) is provided below. A quick note on octahedral geometry: sometimes an octahedral molecule may contain polydentate ligands. Polydentate ligands are ligands that "bite" the central atom in several locations. In other words, polydentate ligands have the ability to form more than one bond with the central metal, unlike other ligands. An example of a polydentate ligand is ethylenediamine, a bidentate ligand, which is abbreviated as en.
SF6:
Applications
Stereoisomers have a lot of applications in biology as well as our day to day lives. Surprisingly, our own tongue contains chiral molecules that help us discern the taste of some of the foods we eat. For example, we may eat two of the same leaves but one may taste bitter and the other may taste sweet because of chirality. As discussed above, optical isomers also have the ability to rotate light in certain directions. In biological terms, chirality is key to the proper functioning of an enzyme. This is because chirality allows the enzyme to function efficiently by being able to bind to only certain substrates.
Problems
- Draw all possible geometric isomers for the tetrahedral molecule MnCl2F2.
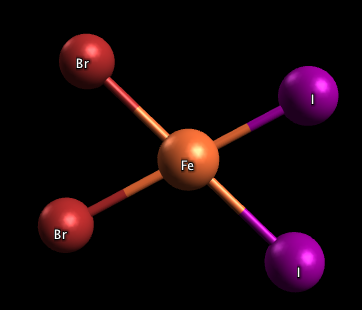
- What type of isomer is the molecule pictured below? Are there any other types of isomers possible for this molecule?
- Draw all possible stereoisomers for the molecule CrF3I3.
- What is the difference between a dextrorotatory optical isomer and a levorotatory optical isomer?
- Draw the molecule Fe(en)3 and state if it is optically active or not.
- True or False: An octahedral molecule can have cis-trans isomers?
Answers
1) MnCl2F2 has no geometric isomers because recall that tetrahedral molecules do not have geometric isomers.
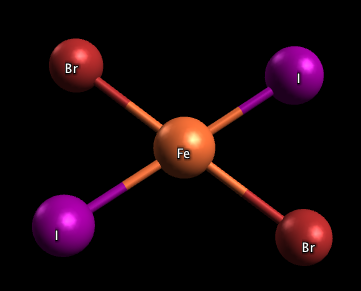
2) The molecule, FeBr2I2 pictured in problem 3 is a cis isomer, or cis-FeBr2I2, because both the Br and I ligands are on the same side. There is one more stereoisomer for this molecule: trans-FeBr2I2, pictured below:
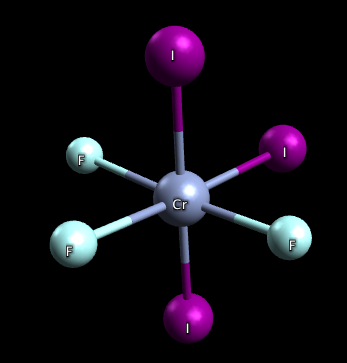
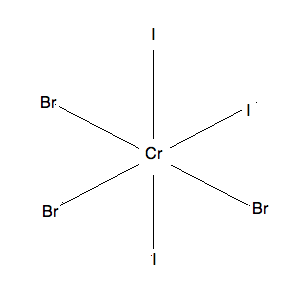
3) This molecule has two isomers: mer-CrF3I3 and fac-CrF3I3, both are pictured below. The mer isomer is where the ligands are not on the same plane and there exists a 90-90-180 degree bond angle between the 3 same ligands. The fac isomer is where the ligands are on the same plane and there exists a 90-90-90 degree bond angle between the 3 same ligands.
fac-CrF3I3:
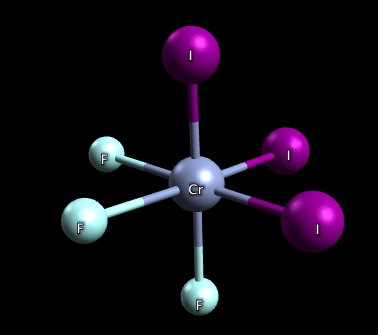
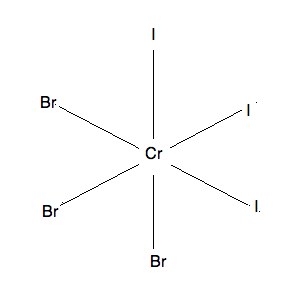
mer-CrF3I3:
4) A dextrorotatory optical isomer is a isomer that can rotate light in the right direction. A levorotatory optical isomer on the other hand is a isomer that can rotate light in the left direction.
5) In the molecule Fe(en)3, recall that the en ligand, ethylenediamine is a bidentate ligand. The 2D structure of this molecule is shown below:
3.png?revision=1&size=bestfit&width=300&height=300)
Note, the black lines represent the bonds and the blue lines represent the bonds ethylenediamine binds to. Using either the symmetry method or the mirror image method, one can observe that this molecule has a chiral center and that the mirror image is not superimposable on the original molecule. Thus, this molecule is optically active because it has optical isomers.
6) True. Octahedral geometry as well as square planar geometry can have cis-trans isomers. The only geometry that cannot have cis-trans isomers is tetrahedral.
References
- Columbia Encyclopedia. "Stereoisomers" in Encyclopedia.com, n.l., 2005, December 2. 2008.
- Petrucci, et al. General Chemistry Principles & Modern Applications. 9th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2007
- 'Inorganic Chemistry' - C. Housecroft and A.G. Sharpe, Prentice Hall, 3rd Ed., Dec 2007, ISBN13: 978-0131755536, ISBN10: 0131755536, Sections 7.11, 7.12 and parts of Chapter 21.
- 'Basic Inorganic Chemistry' - F.A. Cotton, G. Wilkinson and P.L. Gaus, John Wiley and Sons, Inc. 3rd Ed., 1994. pps 165-187, 503-509, 512-517.
- 'Introduction to Modern Inorganic Chemistry' - K.M. Mackay, R.A. Mackay and W. Henderson, International Textbook Company, 5th Ed., 1996.
Contributors
-
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)
- Angad Oberoi (UCD)

