Groupwork 10 Phase equilibria
- Page ID
- 63520
Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help.
A phase diagram depicts the different physical phases possible when a system is in equilibrium. The most familiar phase diagram plots pressure as a function of temperature.
On the phase diagrams below, label the following:
- Where the substance is a gas.
- Where the substance is a liquid
- Where the substance is a solid
- Where the substance can be found either as liquid or gas
- Where the substance can be found either as liquid or solid
- Where the substance can be found as liquid, gas or solid (triple point)
- The critical point
Make sure that the phases you have labeled make sense with respect to the pressure and temperature. That is, what phase do you expect at high temperature and low pressure? At low temperature and high pressure?
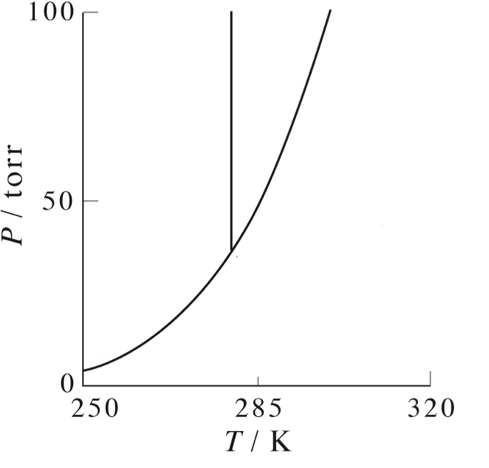
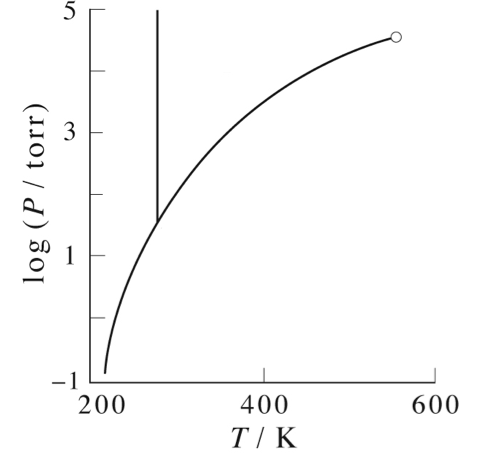
What is the phase of this substance at standard temperature and pressure?
What is the freezing and boiling point for this substance at standard pressure?
Explain what happens as you follow one of the lines on the diagram.

