24.9 Heterocyclic Amines
- Page ID
- 91037
After completing this section, you should be able to
- draw the structure of furan, pyrrole and imidazole.
- use the Hückel 2n + 4 rule to explain the aromaticity of pyrrole.
- predict the product formed when pyrrole is subjected to an aromatic electrophilic substitution, such as nitration, etc.
- write the detailed mechanism for the electrophilic aromatic substitution of pyrrole to account for the fact that substitution takes place at C2 rather than C3.
- describe the geometry of the pyridine molecule.
- account for the difference in basicity between pyridine, pyrrole and other amines.
- explain why pyridine undergoes electrophilic substitution much less readily than does benzene.
- identify the presence of a fused‑ring heterocycle in a given structure.
- make predictions about the chemical behaviour of the fused‑ring heterocycle based on what you have learned about pyrrole, imidazole, pyridine and pyrimidine.
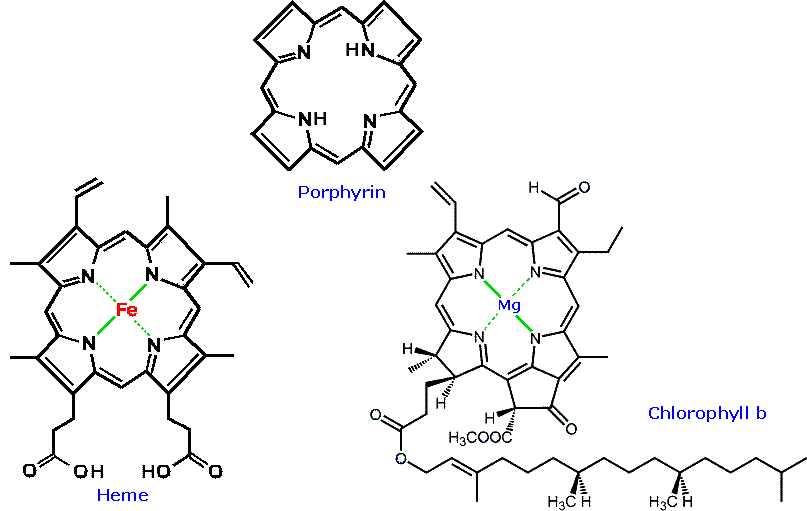
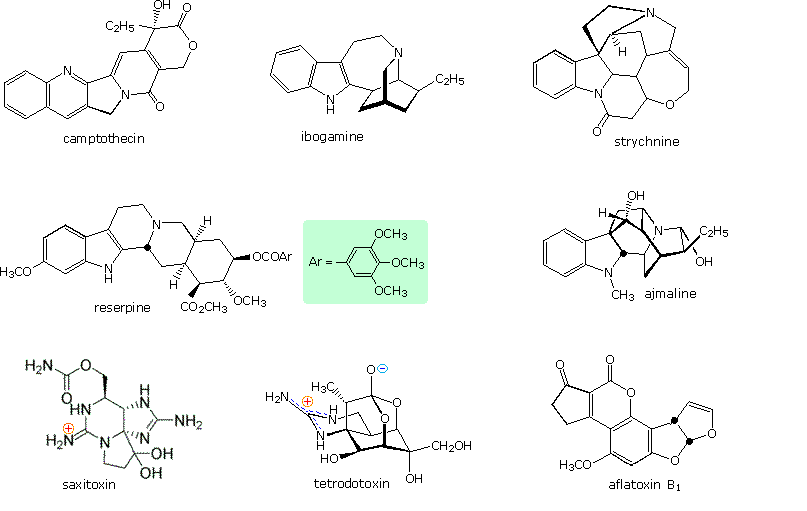
Heterocyclic structures are found in many natural products. Examples of some nitrogen compounds, known as alkaloids because of their basic properties, were given in the amine chapter. Some other examples are displayed in the following diagram. Camptothecin is a quinoline alkaloid which inhibits the DNA enzyme topoisomerase I. Reserpine is an indole alkaloid, which has been used for the control of high blood pressure and the treatment of psychotic behavior. Ajmaline and strychnine are also indole alkaloids, the former being an antiarrhythmic agent and latter an extremely toxic pesticide. The neurotoxins saxitoxin and tetrodotoxin both have marine origins and are characterized by guanidiniun moieties. Aflatoxin B1 is a non-nitrogenous carcinogenic compound produced by the Aspergillus fungus. Porphyrin is an important cyclic tertrapyrrole that is the core structure of heme and chlorophyll.
Pyrrole and Imidazole
Pyrrole is obtained commercially by the reaction of furan with ammomia .
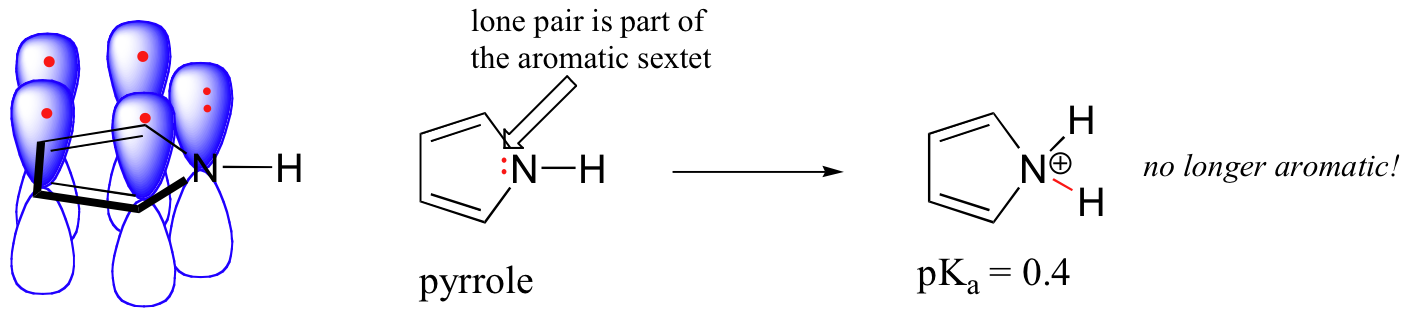
In a pyrrole ring, in contrast, the nitrogen lone pair is part of the aromatic sextet. This means that these electrons are very stable right where they are (in the aromatic system), and are much less available for bonding to a proton (and if they do pick up a proton, the aromic system is destroyed). For these reasons, pyrrole nitrogens are not strongly basic.
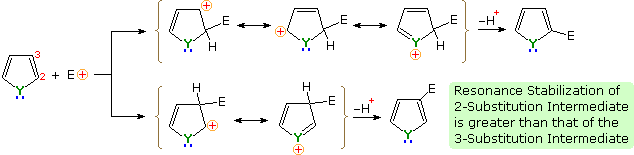
There is a clear preference for substitution at the 2-position (α) of the ring. Reactions of pyrrole require careful evaluation, since N-protonation destroys its aromatic character. Indeed, N-substitution of this 2º-amine is often carried out prior to subsequent reactions. For example, pyrrole reacts with acetic anhydride or acetyl chloride and triethyl amine to give N-acetylpyrrole
An explanation for the general α-selectivity of these substitution reactions is apparent from the mechanism outlined below. The intermediate formed by electrophile attack at C-2 is stabilized by charge delocalization to a greater degree than the intermediate from C-3 attack. From the Hammond postulate we may then infer that the activation energy for substitution at the former position is less than the latter substitution.
Example
Another five-membered heterocyclic amine is imidazole which is part of the amino acid histidine.
Pyridine and Pyrimidine
When a nitrogen atom is incorporated directly into an aromatic ring, its basicity depends on the bonding context. In a pyridine ring, for example, the nitrogen lone pair occupies an sp2-hybrid orbital, and is not part of the aromatic sextet - it is essentially an imine nitrogen. Its electron pair is available for forming a bond to a proton, and thus the pyridine nitrogen atom is somewhat basic.

Fully unsaturated six-membered nitrogen heterocycles, such as pyridine, pyrazine, pyrimidine and pyridazine, have stable aromatic rings. Oxygen and sulfur analogs are necessarily positively charged, as in the case of 2,4,6-triphenylpyrylium tetrafluoroborate.
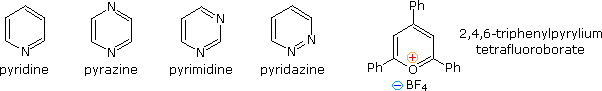
From heat of combustion measurements, the aromatic stabilization energy of pyridine is 21 kcal/mole. The resonance description drawn at the top of the following diagram includes charge separated structures not normally considered for benzene. The greater electronegativity of nitrogen (relative to carbon) suggests that such canonical forms may contribute to a significant degree. Indeed, the larger dipole moment of pyridine compared with piperidine supports this view. Pyridine and its derivatives are weak bases, reflecting the sp2 hybridization of the nitrogen. From the polar canonical forms shown here, it should be apparent that electron donating substituents will increase the basicity of a pyridine, and that substituents on the 2 and 4-positions will influence this basicity more than an equivalent 3-substituent. The pKa values given in the table illustrate a few of these substituent effects. Methyl substituted derivatives have the common names picoline (methyl pyridines), lutidine (dimethyl pyridines) and collidine (trimethyl pyridines). The influence of 2-substituents is complex, consisting of steric hindrance and electrostatic components. 4-Dimethylaminopyridine is a useful catalyst for acylation reactions carried out in pyridine as a solvent. At first glance, the sp3 hybridized nitrogen might appear to be the stronger base, but it should be remembered that N,N-dimethylaniline has a pKa slightly lower than that of pyridine itself. Consequently, the sp2 ring nitrogen is the site at which protonation occurs.
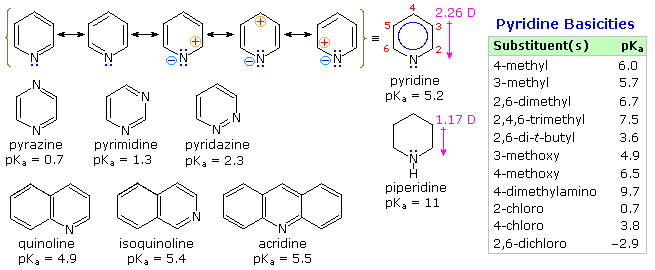
The diazines pyrazine, pyrimidine and pyridazine are all weaker bases than pyridine due to the inductive effect of the second nitrogen. However, the order of base strength is unexpected. A consideration of the polar contributors helps to explain the difference between pyrazine and pyrimidine, but the basicity of pyridazine seems anomalous. It has been suggested that electron pair repulsion involving the vicinal nitrogens destabilizes the neutral base relative to its conjugate acid
From the previous resonance description of pyridine, we expect this aromatic amine to undergo electrophilic substitution reactions far less easily than does benzene. Furthermore, the electrophilic reagents and catalysts employed in these reactions coordinate with the nitrogen electron pair, exacerbating the positive charge at positions 2,4 & 6 of the pyridine ring. Three examples of the extreme conditions required for electrophilic substitution are shown below.
Polycyclic Heterocycles
Polycyclic Heterocyclic structures are found in many natural products.
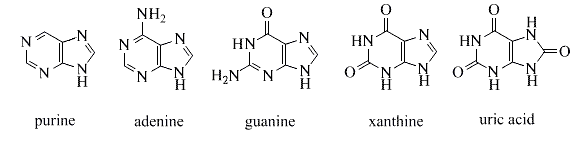
Derivatives of the simple fused ring heterocycle purine constitute an especially important and abundant family of natural products. The amino compounds adenine and guanine are two of the complementary bases that are essential components of DNA. Structures for these compounds are shown in the following diagram. Xanthine and uric acid are products of the metabolic oxidation of purines. Uric acid is normally excreted in the urine; an excess serum accumulation of uric acid may lead to an arthritic condition known as gout.
The fused ring heterocycles quinoline and isoquinoline provide additional evidence for the stability of the pyridine ring. Vigorous permanganate oxidation of quinoline results in predominant attack on the benzene ring; isoquinoline yields products from cleavage of both rings.
Indole has a nonbasic nitrogen similar to to pyrrole. It undergo electrophilic substitution more easily than benzene and substitution typically occurs at the C3 position on the pyrole ring.
Exercises
Describe how thiazole is aromatic. Use an orbital picture and include the lone pair electrons on sulfur. Assume the sulfur is sp2 hybridized.
- Answer
-

Would you expect a thiazole ring to be protonated at the physiological pH of 7.3 (Section 24-5)?
- Answer
-
7.3 = 2.44 + log ([RNH2] / [RNH3+])
([RNH2] / [RNH3+]) = 7.2 x 104
. . . so, [RNH2] >> [RNH3+] so thiazole would be almost completely unprotonated at pH = 7.3.
Which nitrogen atom in the neurotransmitter serotonin is expected to be the most basic? Please explain your answer.
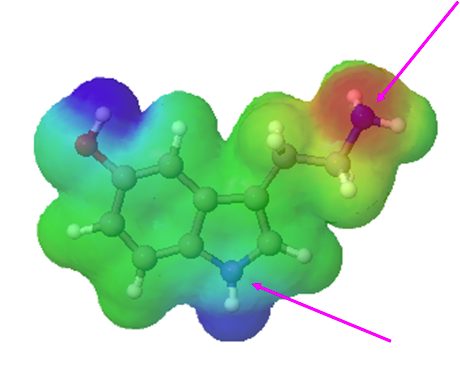
- Answer
-
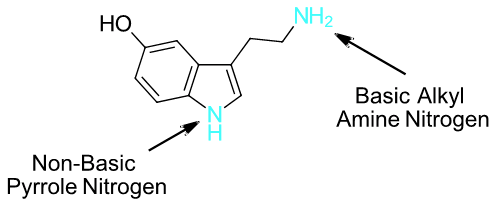
Pyridine reacts with electrophiles to product a 3-substituted product rather than a 2-substituted product. Write a series of resonance forms for the cation intermediate formed during the reaction. Use these structures to explain the experimental result.
- Answer
-
The resonance forms of 2-substitution places a positive charge on the ring nitrogen. The resonance forms of 3-substitution do not place a positive charge on the ring nitrogen. Having a positive charge on a nitrogen is less stable than on a carbon. Nitrogen is more electronegative than carbon making it less able to stabilize the positive charge. The 3-substituted product is preferred because it causes resonance forms which are more stable.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry