23.10 Conjugate Carbonyl Additions - The Michael Reaction
- Page ID
- 91023
Objectives
After completing thissection, you should be able to
- write an equation to illustrate the Michael reaction.
- write a detailed mechanism for a given typical Michael reaction.
- identify the product formed in a given Michael reaction.
- identify the reagents necessary to synthesize a given compound by a Michael reaction.
- Michael reaction
Before studying this section in detail, you should review conjugate addition to α,β‑unsaturated carbonyl compounds (Section 19.13).
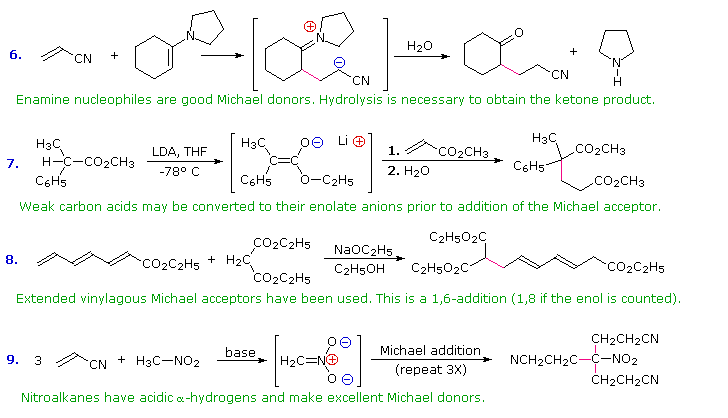
Notice the wide variety of compounds that can take part in a Michael reaction. As usual, you should not feel overwhelmed by the number of different compounds that can be used, just keep in mind that in all cases the mechanism is essentially the same.
Basic reaction of 1,4 addition
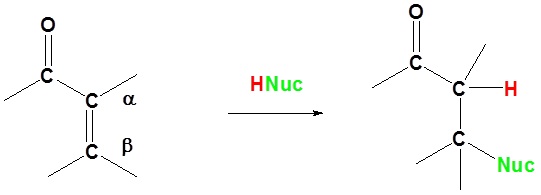
In 1,4 addition the Nucleophile is added to the carbon β to the carbonyl while the hydrogen is added to the carbon α to the carbonyl.
Mechanism for 1,4 addition
1) Nucleophilic attack on the carbon β to the carbonyl
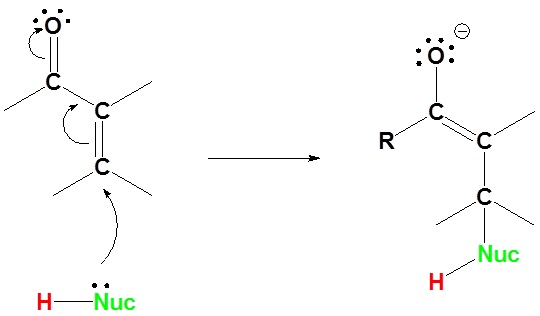
2) Proton Transfer
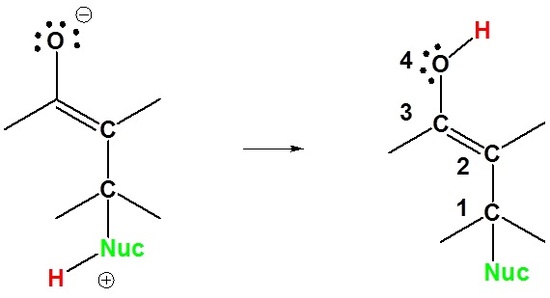
Here we can see why this addition is called 1,4. The nucleophile bonds to the carbon in the one position and the hydrogen adds to the oxygen in the four position.
3) Tautomerization
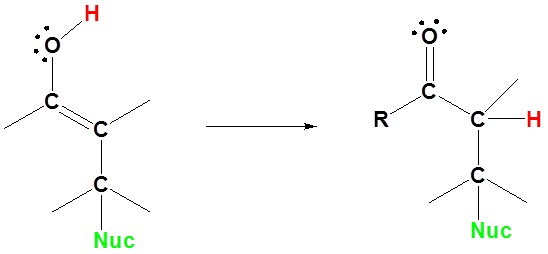
Enolates undergo 1,4 addition to α, β-unsaturated carbonyl compounds is a process called a Michael addition. The reaction is named after American chemist Arthur Michael (1853-1942).
Examples of Michael Additions
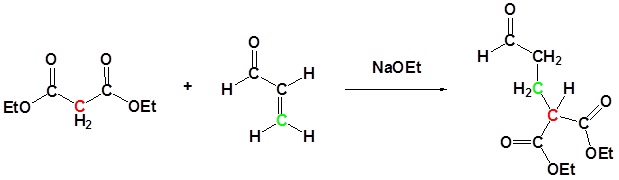
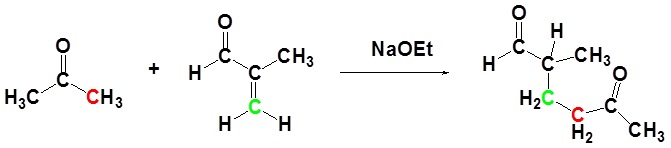
In combination with alkylations and condensations, the Michael reaction may be used to construct a wide variety of complex molecules from relatively simple starting materials. The carbon nucleophiles used in the following examples include cyanide ion, sodium diethylmalonate and the conjugate base of cyclohexane-1,3-dione. These anions are sufficiently stable that their addition reactions may be presumed reversible. If this is so, the thermodynamic argument used for hetero-nucleophile additions would apply here as well, and would indicate preferential formation of 1,4-addition products. Cyanide addition does not always follow this rule, and aldehydes often give 1,2-products (cyanohydrins). In each case the initial reaction is a Michael addition, and the new carbon-carbon bond is colored magenta. Any subsequent bonds that are formed by other reactions are colored orange.
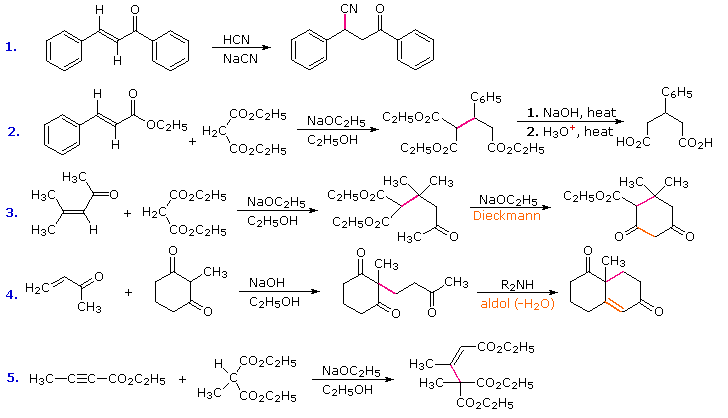
Exercises
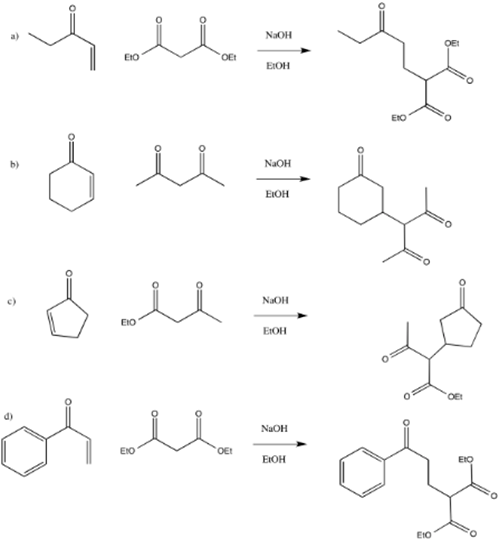
Provide the products of the following Michael additions:

- Answer
-
What would be the starting materials required to make the following molecule using a Michael reaction? Red = Oxygen, Grey = Carbon, White = Hydrogen, Blue = Nitrogen.
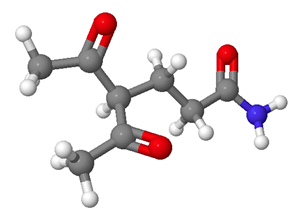
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry