22.6 Reactivity of Enolate Ions
- Page ID
- 91010
Objectives
After completing this section, you should be able to
- identify the two possible ways in which a given enolate anion could conceivably react with an electrophile.
- write an equation to illustrate the haloform reaction.
- identify the products formed from the reaction of a given methyl ketone with a halogen and excess base.
- identify the methyl ketone, the reagents, or both, needed to obtain a specified carboxylic acid through a haloform reaction.
Make certain that you can define, and use in context, the key terms below.
- haloform
- haloform reaction
Because the negative charge on an enolate ion is delocalized, there are two reactive sites and therefore two potential products. The α‑substituted product is much more common.
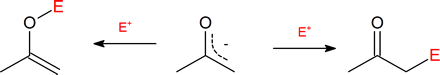
A “haloform” is any compound of the type CHX3, where X = Cl, Br or I. Of these three compounds, chloroform is the most common.
The haloform reaction describedin the reading is usually carried out with iodine. This reaction is called the “iodoform test,” and is one of the reactions carried out in the laboratory as a simple qualitative test for methyl ketones.
How Enolates React
Due to their negative charges, enolates are better and more versatile nucleophiles than enols. The increased reactivity of enolates makes them capable of a wider range of reactions than enols. Also, α-hydrogen containing compounds can be completely converted to an enolate by reaction with a strong base. Whereas enols can only be created in small amounts through manipulating their equilibrium.
Since the negative charge of an enolate anion is delocalized between the α-carbon and an oxygen, electrophiles may bond to either atom. Reactants having two or more reactive sites are called ambident, so this term applies to enolate anions. Either the C of the O reactive site in an enolate may act as a nucleophile depending on the reaction conditions. Reactions with the oxygen would create a new O-E bond and produce an enol derivative. Reactions with the α-carbon creates a new C-E bond and creates an α-substituted carbonyl compound. Although reactions with the nucleophilic oxygen are possible, reactions involving the nucleophilic α-carbon are much more common, partially due to the thermodynamic stability of the C=O bonds in the final products. Also, the enolate counter ion, such as Li+ or Na+, is more tightly associated with the negatively charged enolate oxygen which can then block incoming electrophiles, reducing their chance of reaction at the oxygen.
Stereochemical Implication of Enolate Formation
During enolate formation, an α-hydrogen is removed to form a sp2-hybridized, trigonal planar C=C bond which removes any chiral information from the original α-carbon. Because the enol alkene is planar, the incoming electrophile can attack from either the top or the bottom. If the α-carbon of the starting material has a defined stereochemistry or if a new stereocenter is formed during the reaction, the product will be a racemic mixture of enantiomers.
Base Promoted α-Halogenation
An enolate reacts rapidly with a halogen to produce α-halogenated carbonyl products. This reaction has the tendency to overreact and create polyhalogenated products. If a monohalogenated product is sought, the acid catalyzed halogenation reaction discussed in section 22.3 is preferred. Because complete formation to the enolate is not necessary, weak bases, such as the hydroxide anion, are sufficient to produce this reaction. Once a small amount of enolate is formed, it quickly reacts with the halogen. This removes the enolate and shifts the equilibrium toward forming more enolate by Le Chatelier's principle.
Overreaction During Base Promoted α-Halogenation
The α-hydrogens of halogenated carbonyl products are usually more acidic than the corresponding non-halogenated compounds. The inductive electron withdrawing effect of the electronegative halogen stabilizes the negative charge of the enolate ion. This promotes further enolate formation and also further halogenation of the α-carbon. Monohalogenated carbonyls form an enolate over 100 times faster than their non-halogenated counterparts making multiple halogenations of the α-carbon frequent. This effect is exploited to cause the haloform reaction.
The Haloform Reaction
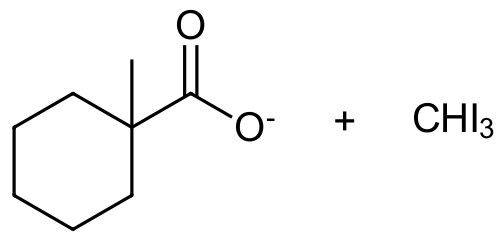
Overall, the haloform reaction represents a method for the conversion of methyl ketones to carboxylic acids. Due to the increased reactivity of α-halogenated products, methyl ketones typically undergo base promoted halogenation three times to give a trihalo-ketone. A halomethyl ion leaving group is then substituted with a hydroxide ion during nucleophilic acyl substitution. The resulting carboxylate can then be protonated to form a carboxylic acid.
Example
Mechanism
Note! This reaction is considered to be base promoted and not base catalyzed because an entire equivalent of base is required for each α-halogenation. Deprotonation of an α-hydrogen with hydroxide produces the nucleophilic enolate ion which subsequently reacts with the halogen. The increasing acidity of α-halogenated ketone causes this reaction to occur two more times. Once formed, the -CX3 group attached to the carbonyl can act as a leaving group. Nucleophilic acyl substitution with a hydroxide anion causes C-C bond cleavage and eventually produces a haloform (CHCl3, CHBr3, CHI3) and a carboxylate anion. The carboxylate ion is easily protonated with acid to form a carboxylic acid functional group. Often, this reaction is performed using iodine (I2) because the subsequent iodoform (CHI3) side-product is a bright yellow solid which is easily filtered off.
This reaction represents one of the few examples of a carbanion leaving group. The trihalomethyl ion (-:CX3) is particularly stabilized due to the inductive electron-withdrawing effects of the three halogens. The stability of the carbanion can be seen when considering the pKa corresponding conjugate acid. In particular, bromoform (CHBr3) has a pKa of 13.7 which is more than 1020 times more acidic than a typical alkane C-H bond.
1) Enolate formation
2) Nucleophilic attack on the halogen
3) Repeat the halogenation two more times
4) Nucleophilic attack on the electrophilic carbonyl carbon
5) Nucleophilic acyl substitution
6) Deprotonation
7) Protonation of the carboxylate
Exercises
Please predict the expected products of the following reaction.
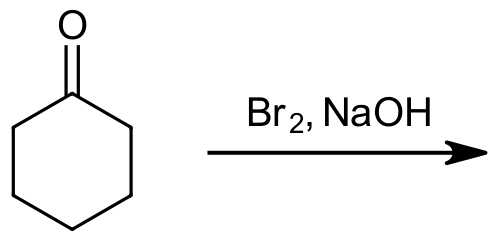
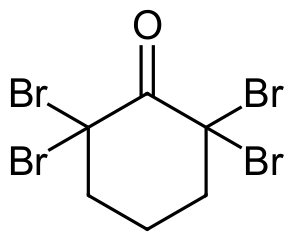
- Answer
-
Please predict the expected products of the following reaction.
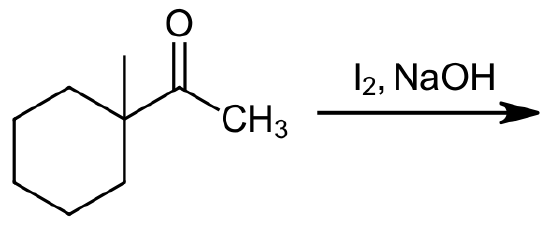
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

