21.7 Chemistry of Amides
- Page ID
- 90998
Objectives
After completing this section, you should be able to
- write an equation to describe the preparation of an amide from an acid chloride.
- identify the amide linkage as the basic unit from which all proteins are made, and hence recognize the importance of the amide linkage to biologists and biochemists.
- write detailed mechanisms for the acidic and basic hydrolysis of amides.
- write an equation to describe the reduction of an amide to an amine.
- write a detailed mechanism for the reduction of an amide to an amine.
- identify the product formed when a given amide is reduced with lithium aluminum hydride.
- identify the amide, the reagents, or both, necessary to prepare a given amine by direct reduction.
- identify lactams as being cyclic amides which undergo hydrolysis and reduction in a manner analogous to that of their acyclic counterparts.
Make certain that you can define, and use in context, the key term below.
- lactam
As the chapter which deals with amino acids and proteins is optional, it is possible for you to complete this course without studying these compounds in detail. However, because of their importance in biological systems, it is essential for all students completing this course to have some knowledge of their structure and properties.
When we talk about amino acids, we are generally referring to α‑amino acids; that is, compounds in which an amino (NH2) group and a carboxyl group are attached to the same carbon atom:
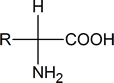
Notice that such compounds contain a chiral carbon atom (unless R = H). Proteins can be considered to consist of amino acid residues joined by amide (or peptide) links. These peptide links consist of exactly the same structural units that we find in secondary amides
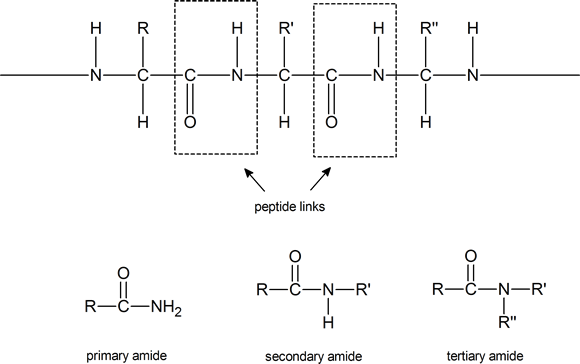
Note: Although the terms secondary amide and tertiary amide have been used here, this usage is not in agreement with IUPAC recommendations. According to IUPAC, the secondary amide shown above should be referred to as an N‑substituted primary amide, and the tertiary amide should be referred to as a N, N‑disubstituted primary amide. IUPAC reserves the term secondary amide for compounds of the type
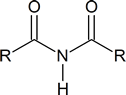
Because of the presence of the amide link in proteins,
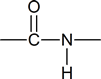
we can expect the properties of these compounds to resemble those of secondary amides.
A lactam is a cyclic amide, in the same way that a lactone is a cyclic ester:
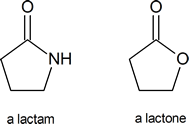
Among the most important molecules that contain lactam rings are the penicillins:
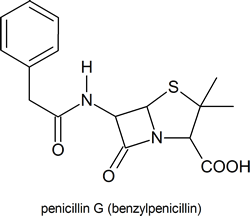
Preparation of Amides
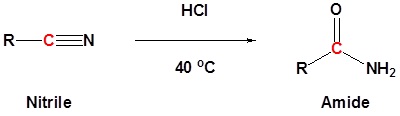
Nitriles can be converted to amides. This reaction can be acid or base catalyzed
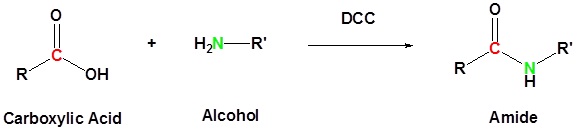
Carboxylic acid can be converted to amides by using DCC as an activating agent
Direct conversion of a carboxylic acid to an amide by reaction with an amine.
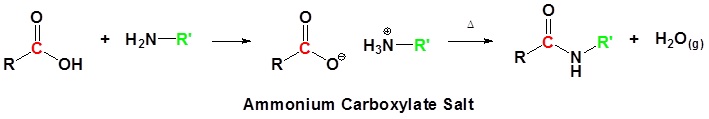
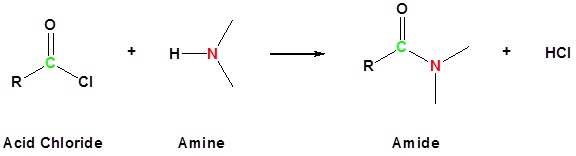
Acid chlorides react with ammonia, 1o amines and 2o amines to form amides
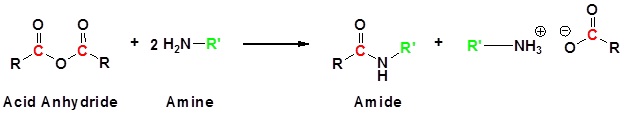
Acid Anhydrides react with ammonia, 1o amines and 2o amines to form amides
Conversion of Amides into Carboxylic Acids: Hydrolysis
This page describes the hydrolysis of amides under both acidic and alkaline conditions. It also describes the use of alkaline hydrolysis in testing for amides.
What is hydrolysis?
Technically, hydrolysis is a reaction with water. That is exactly what happens when amides are hydrolyzed in the presence of dilute acids such as dilute hydrochloric acid. The acid acts as a catalyst for the reaction between the amide and water. The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis.
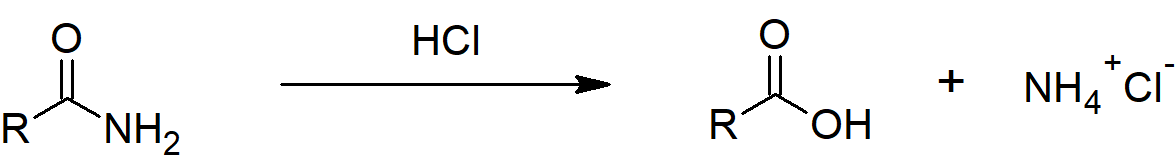
Hydrolysis under acidic conditions
Taking ethanamide as a typical amide. If ethanamide is heated with a dilute acid (such as dilute hydrochloric acid), ethanoic acid is formed together with ammonium ions. So, if you were using hydrochloric acid, the final solution would contain ammonium chloride and ethanoic acid.
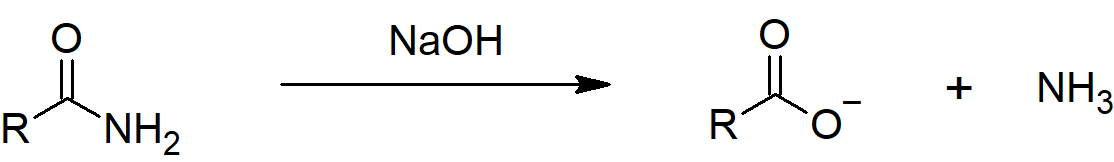
Hydrolysis under alkaline conditions
Also, if ethanamide is heated with sodium hydroxide solution, ammonia gas is given off and you are left with a solution containing sodium ethanoate.
Conversion of Amides into Amines: Reduction
Amides can be converted to 1°, 2° or 3° amines using LiAlH4.
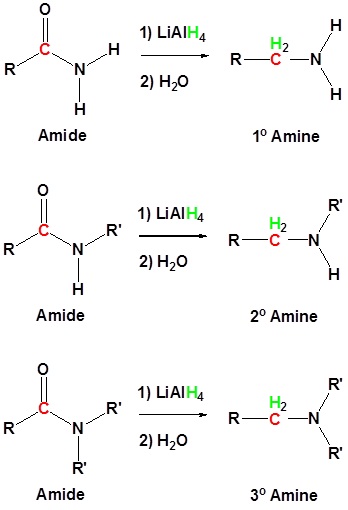
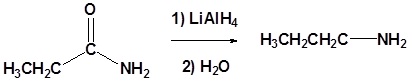
Alkyl groups attached to the nitrogen do not affect the reaction.
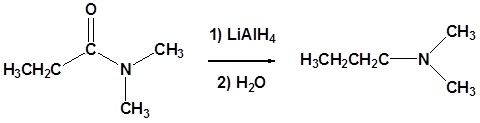
Mechanism
1) Nucleophilic attach by the hydride
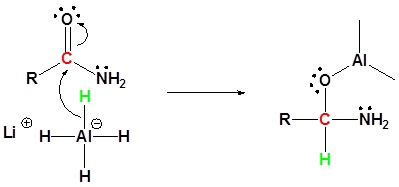
2) Leaving group removal
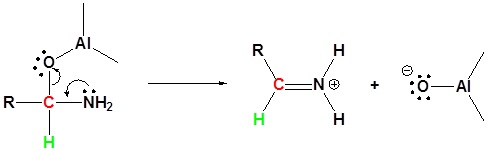
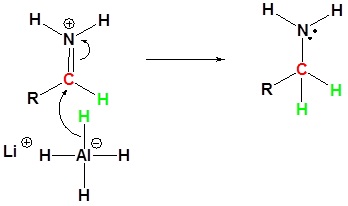
3) Nucleophilic attach by the hydride
How could the following molecule be synthesized using an aminolysis of an acid chloride?
- Answer
-
One of the preferred methods for making amines is through a nucleophilic acyl substitution using an acid chloride and amine to form an amide. The amide is then reduced to the amine during a hydride reduction with LiAlH4.
In the first step of retrosynthetic analysis, one of the carbons alpha to the amine nitrogen is converted to a carbonyl thus creating an amide intermediate. The key bond formed during this pathway is the C-N sigma bond between the carbonyl carbon and the nitrogen created during amide formation. Breaking this bond separated the target molecule into the two starting materials for the pathway. The carbonyl carbon gains a –Cl to become an acid chloride and the nitrogen fragment gains an H to become a 1o amine. In the forward reaction pathway, the acid chloride and the 1o amine are linked to from an amide in the first step of the reaction. Subsequent hydride reduction of the amide using LiAlH4 produces the amine target molecule.
The target molecule has two alpha carbons which could possibly be converted to a carbonyl. This allows there to be two possible synthetic pathways.
Pathway 1
Solution 1
Pathway 2
Solution 2
Exercises
How would you prepare the following compounds from N-Propypl benzamide?
(a)
(b)
(c)
- Answer
-
(a) NaOH, H2O
(b) NaOH, H2O, then LiAlH4
(c) LiAlH4
Propose a synthesis pathway for the following conversion:
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)

