21.0 Introduction
- Page ID
- 90991
After completing this section, you should be able to
- identify, and give an example of
- an acid halide.
- an acid anhydride.
- an ester.
- an amide.
- write the general form of a nucleophilic acyl substitution reaction.
Make certain that you can define, and use in context, the key terms below.
- acyl derivative
- nucleophilic acyl substitution reaction
Carboxylic Acid Derivatives
A acyl derivative (functional derivative) of a carboxylic acid is a substance formed by replacement of the hydroxyl group of the acid by some other group, \(\ce{X}\), such that it can be hydrolyzed back to the acid in accord with Equation 18-7:
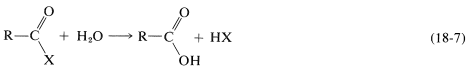
By this definition, an amide, \(\ce{RCONH_2}\), but not a ketone, \(\ce{RCOCH_3}\), is a functional derivative of a carboxylic acid. Several derivatives of carboxylic acids are give in Table 1.
Table 1: Functional Derivatives of Carboxylic Acids
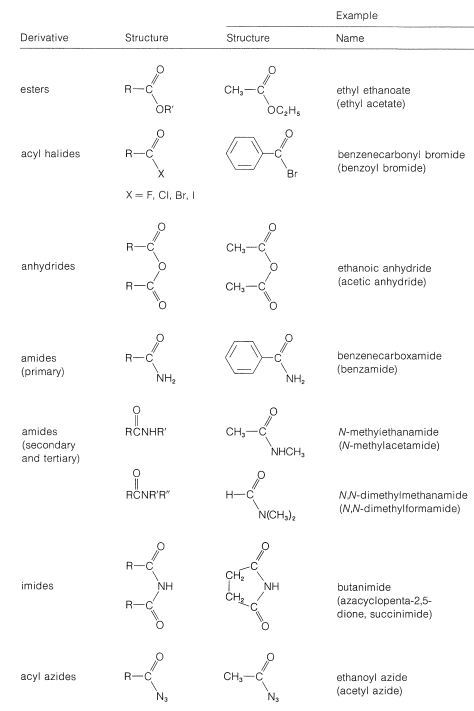
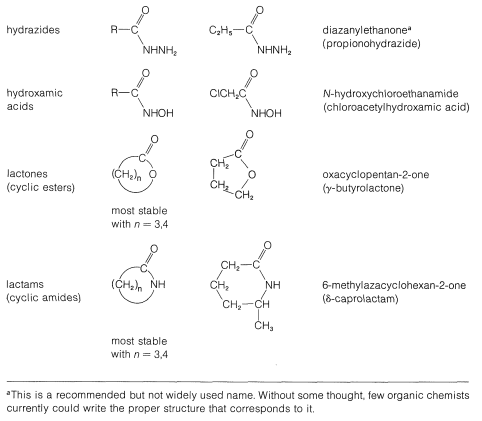
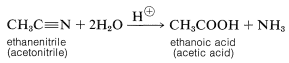
The common structural feature of the compounds listed in Table 3 is the acyl group \(\ce{RCO}-\). However, nitriles, \(\ce{RC \equiv N}\), often are considered to be acid derivatives, even though the acyl group is not present as such, because hydrolysis of nitriles leads to carboxylic acids:
Nucleophilic Acyl Substitution Reaction
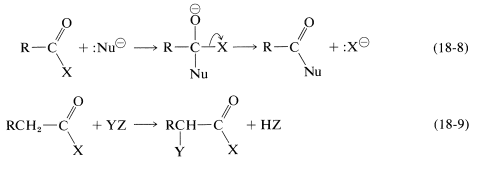
The main type of reaction of carboxylic acid derivatives with which we now shall be concerned are the replacement of \(\ce{X}\) by attack of a nucleophile \(\ce{Nu}^\ominus\) at the carbonyl carbon with subsequent cleavage of the \(\ce{C-X}\) bond (Equation 18-8) or an nucleophilic acyl substitution reaction. Carboxylic acid derivatives can also undergo substitution at the \(\alpha\) carbon facilitated by the carbonyl group (Equation 18-9):
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."

