19.14 Spectroscopy of Aldehydes and Ketones
- Page ID
- 90977
Objectives
After completing this section, you should be able to
- identify the region of the infrared spectrum in which the carbonyl absorption of aldehydes and ketones is found.
- identify the region of the infrared spectrum in which the two characteristic C$\ce{-}$H absorptions of aldehydes are found.
- use a table of characteristic absorption frequencies to assist in the determination of the structure of an unknown aldehyde or ketone, given its infrared spectrum and other spectral or experimental data.
- identify the region of a proton NMR spectrum in which absorptions caused by the presence of aldehydic protons and protons attached to the α‑carbon atoms of aldehydes and ketones occur.
- identify two important fragmentations that occur when aliphatic aldehydes and ketones are subjected to analysis by mass spectrometry.
Make certain that you can define, and use in context, the key term below.
- McLafferty rearrangement
The appearance of a strong absorption at 1660–1770 cm−1 in the infrared spectrum of a compound is a clear indication of the presence of a carbonyl group. Although you need not remember the detailed absorptions it is important that you realize that the precise wavenumber of the infrared absorption can often provide some quite specific information about the environment of the carbonyl group in a compound. Notice how conjugation between a carbonyl group and a double bond (α, β‑unsaturated aldehyde or ketone or aromatic ring) lowers the absorption by about 25–30 cm−1.
You may wish to review the McLafferty rearrangement and the alpha cleavage in Section 12.3.
IR Spectra
The carbonyl stretching vibration band C=O of saturated aliphatic ketones appears:
C=O stretch
- aliphatic ketones 1715 cm-1
- alpha, beta-unsaturated ketones 1685-1666 cm-1
Figure 8. shows the spectrum of 2-butanone. This is a saturated ketone, and the C=O band appears at 1715.
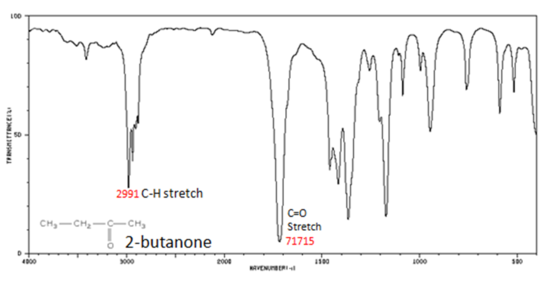
Figure 8. Infrared Spectrum of 2-Butanone
If a compound is suspected to be an aldehyde, a peak always appears around 2720 cm-1 which often appears as a shoulder-type peak just to the right of the alkyl C–H stretches.
H–C=O stretch 2830-2695 cm-1
C=O stretch
- aliphatic aldehydes 1740-1720 cm-1
- alpha, beta-unsaturated aldehydes 1710-1685 cm-1
Figure 9. shows the spectrum of butyraldehyde.
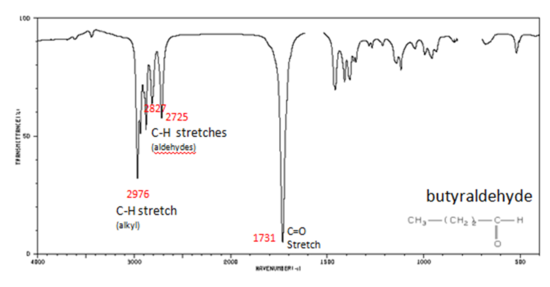
Figure 9. Infrared Spectrum of Butyraldehyde
NMR Spectra
Hydrogens attached to carbon adjacent to the sp2 hybridized carbon in aldehydes and ketones usually show up 2.0-2.5 ppm.
.
Aldehyde hydrogens are highly deshielded and appear far downfield as 9-10 ppm.
Chemical shift of each protons is predicted by 1H chemical shift ranges (Ha): chemical shift of methyl groups (1.1 ppm). (Hb) The chemical shift of the -CH- group move downfield due to effect an adjacent aldehyde group: (2.4 ppm). The chemical shift of aldehyde hydrogen is highly deshielded (9.6 ppm).

4) Splitting pattern is determined by (N+1) rule: Ha is split into two peaks by Hb(#of proton=1). Hb has the septet pattern by Ha (#of proton=6). Hc has one peak.(Note that Hc has doublet pattern by Hb due to vicinal proton-proton coupling.)
Mass Spectra
Aldehydes and ketones generally give moderately intense signals due to their molecular ions, \(\ce{M^+}\). Thus the determination of the molecular weight of a ketone by mass spectroscopy usually is not difficult. Furthermore, there are some characteristic fragmentation patterns that aid in structural identification. These are:
- Formation of the molecular ion:
- \(\alpha\) cleavage to form an acylium ion. This is usually the base peak in the mass spectra.
- A common fragmentation pattern for larger carbonyl compounds is a transfer of \(\gamma\) hydrogen with \(\beta\) cleavage called the McLafferty rearrangement:
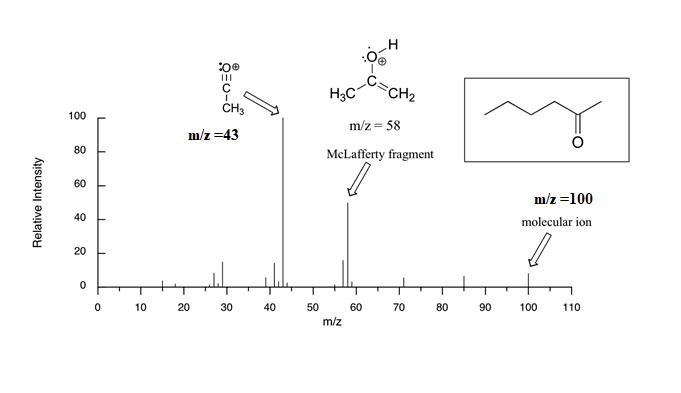
The mass spectrum of 2-hexanone shows a 'McLafferty fragment' at m/z = 58, while the propene fragment is not observed because it is a neutral species (remember, only cationic fragments are observed in MS). The base peak in this spectrum is an acylium ion formed after alpha cleavage is at m/z = 43. The molecular ion peak is at m/z =100.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

