19.13 Conjugate Nucleophilic Addition to alpha, beta-unsaturated Aldehydes and Ketones
- Page ID
- 90976
Objectives
After completing this section, you should be able to
- explain how the carbonyl group which is present in α, β‑unsaturated aldehydes and ketones activates the carbon‑carbon double bond so that it is susceptible to attack by nucleophiles.
- write equations to illustrate the addition of amines, water and lithium diorganocopper reagents to α, β‑unsaturated aldehydes and ketones.
- identify the product formed from the reaction of a given primary or secondary amine with a given α, β‑unsaturated aldehyde or ketone.
- identify the aldehyde or ketone, the primary or secondary amine, or both, needed to prepare a given β‑amino aldehyde or ketone.
- identify the product formed from the reaction of an α, β‑unsaturated aldehyde or ketone with water.
- identify the product formed from the reaction of a given α, β‑unsaturated aldehyde or ketone with a given lithium diorganocopper reagent.
- identify the α, β‑unsaturated aldehyde or ketone, the lithium diorganocopper reagent, or both, needed to prepare a given product through a conjugate addition reaction.
At first this section may appear to contain a considerable amount of information, but you should realize that much of the material presented is really repetition. Essentially we see how three different nucleophilic reagents, primary and secondary amines, water and lithium diorganocoppers can add across a carbon‑carbon double bond when the latter is conjugated to the carbonyl group of an aldehyde or ketone. Note that the first reagent can also react directly with the carbonyl group of an aldehyde or ketone when there is no conjugated carbon‑carbon double bond present, but that the third reagent, lithium dialkylcopper, cannot do so.
You may be confused about the designation of the product from the conjugate addition to an α, β‑unsaturated aldehyde or ketone as a 1,4 adduct. You can more clearly understand this name if you recognize that the proton added in the second step of the reaction first adds to the oxygen of the enolate ion to produce an enol. The latter then tautomerizes to the more stable keto form.
One of the largest and most diverse classes of reactions is composed of nucleophilic additions to a carbonyl group. Conjugation of a double bond to a carbonyl group transmits the electrophilic character of the carbonyl carbon to the beta-carbon of the double bond. These conjugated carbonyl are called enones or α, β unsaturated carbonyls. A resonance description of this transmission is shown below.
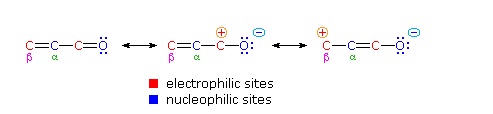
From this formula it should be clear that nucleophiles may attack either at the carbonyl carbon, as for any aldehyde, ketone or carboxylic acid derivative, or at the beta-carbon. These two modes of reaction are referred to as 1,2-addition and 1,4-addition respectively. A 1,4-addition is also called a conjugate addition.
Basic reaction of 1,2 addition
Here the nucleophile adds to the carbon which is in the one position. The hydrogen adds to the oxygen which is in the two position.
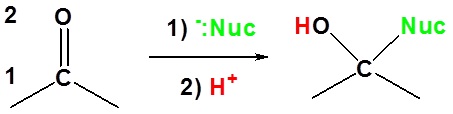
Basic reaction of 1,4 addition
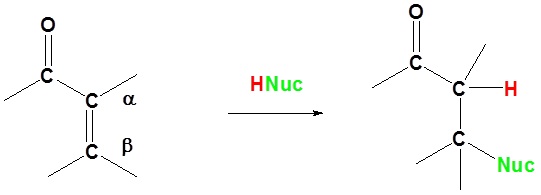
In 1,4 addition the Nucleophile is added to the carbon β to the carbonyl while the hydrogen is added to the carbon α to the carbonyl.
Mechanism for 1,4 addition
1) Nucleophilic attack on the carbon β to the carbonyl
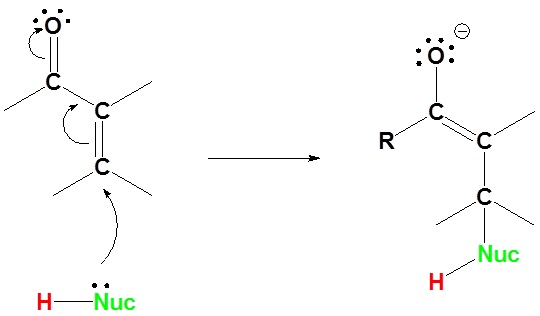
2) Proton Transfer
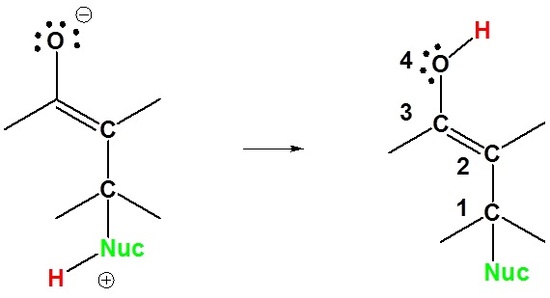
Here we can see why this addition is called 1,4. The nucleophile bonds to the carbon in the one position and the hydrogen adds to the oxygen in the four position.
3) Tautomerization
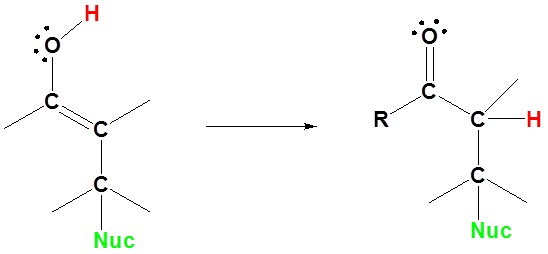
Going from reactant to products simplified
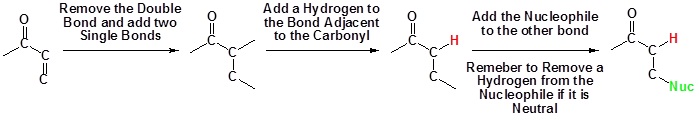
1,2 Vs. 1,4 addition
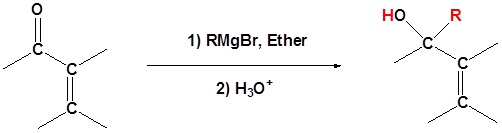
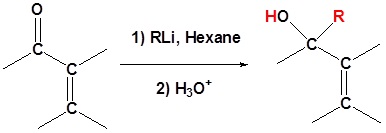
Whether 1,2 or 1,4-addition occurs depends on multiple variables but mostly it is determined by the nature of the nucleophile. During the addition of a nucleophile there is a competition between 1,2 and 1,4 addition products. If the nucleophile is a strong base, such as Grignard reagents, both the 1,2 and 1,4 reactions are irreversible and therefor are under kinetic control. Since 1,2-additions to the carbonyl group are fast, we would expect to find a predominance of 1,2-products from these reactions.
If the nucleophile is a weak base, such as alcohols or amines, then the 1,2 addition is usually reversible. This means the competition between 1,2 and 1,4 addition is under thermodynamic control. In this case 1,4-addition dominates because the stable carbonyl group is retained.
Nucleophiles which add 1,4 to α, β unsaturated carbonyls
Water
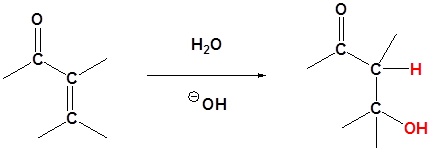
Alcohols
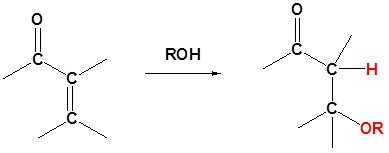
Thiols
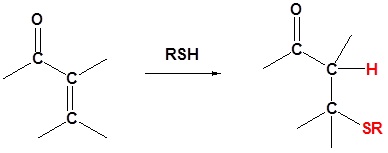
1o Amines
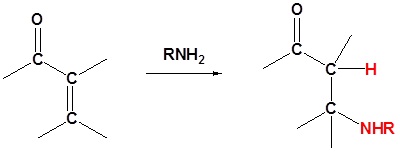
2o Amines
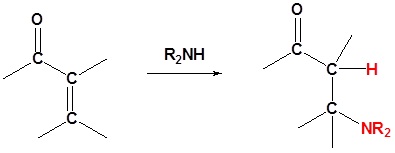
HBr
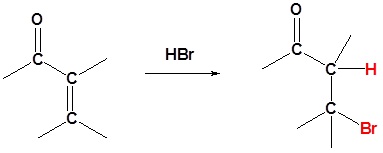
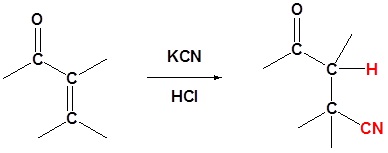
Cyanides
Gilman Reagents
Another important reaction exhibited by organometallic reagents is metal exchange. Organolithium reagents react with cuprous iodide to give a lithium dimethylcopper reagent, which is referred to as a Gilman reagent. Gilman reagents are a source of carbanion like nucleophiles similar to Grignard and Organo lithium reagents. However, the reactivity of organocuprate reagents is slightly different and this difference will be exploited in different situations. In the case of α, β unsaturated carbonyls organocuprate reagents allow for an 1,4 addition of an alkyl group. As we will see later Grignard and Organolithium reagents add alkyl groups 1,2 to α, β unsaturated carbonyls
Organocuprate reagents are made from the reaction of organolithium reagents and CuI

This acts as a source of R:-

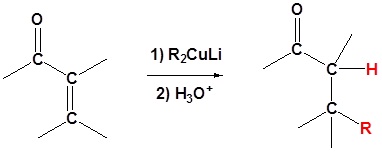
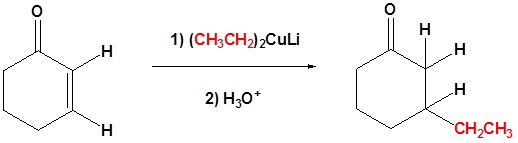
Example
Nucleophiles which add 1,2 to α, β unsaturated carbonyls
Metal Hydrides
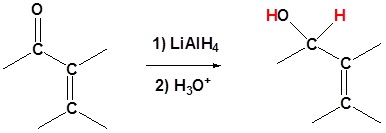
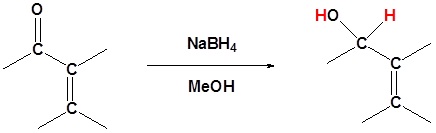
Grignard Reagents
Organolithium Reagents
Exercises
How would you make the following molecule using a 1,4 Conjugate Addition of a Gilman Reagen to a α, β Unsaturated Ketone?
- Answer
-
Draw the bond-line structures for the products of the reactions below.
a)
b)
- Answer
-
a)
b)
Specify the reagents needed to perform the following chemical transformation.
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

