17.7 Oxidation of Alcohols
- Page ID
- 90944
Objectives
After completing this section, you should be able to:
- write an equation to represent the oxidation of an alcohol.
- identify the reagents that may be used to oxidize a given alcohol.
- identify the specific reagent that is used to oxidize primary alcohols to aldehydes rather than to carboxylic acids.
- identify the product formed from the oxidation of a given alcohol with a specified oxidizing agent.
- identify the alcohol needed to prepare a given aldehyde, ketone or carboxylic acid by simple oxidation.
- write a mechanism for the oxidation of an alcohol using a chromium(VI) reagent.
The reading mentions that pyridinium chlorochromate (PCC) is a milder version of chromic acid that is suitable for converting a primary alcohol into an aldehyde without oxidizing it all the way to a carboxylic acid. This reagent is being replaced in laboratories by Dess‑Martin periodinane (DMP), which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous reaction conditions. DMP is named after Daniel Dess and James Martin, who developed it in 1983.

This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate(VI) solution. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a way of distinguishing between primary, secondary and tertiary alcohols.
Oxidizing the different types of alcohols
The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromate(VI) acidified with dilute sulfuric acid. If oxidation occurs, the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions. The electron-half-equation for this reaction is:
\[ Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O \tag{17.7.1}\]
Oxidizing agents
- K2Cr2O7 potassium dichromate
- CrO3 Chromium Trioxide
Both of these are used along with H2SO4, H2O
- 1o alcohol → Carboxylic acid
- 2o alcohol → Ketone
- 3o alcohol → No reaction
Primary alcohols
Primary alcohols can be oxidized to either aldehydes or carboxylic acids depending on the reaction conditions. In the case of the formation of carboxylic acids, the alcohol is first oxidized to an aldehyde which is then oxidized further to the acid.
Full oxidation to carboxylic acids
You need to use an excess of the oxidizing agent and make sure that the aldehyde formed as the half-way product stays in the mixture. The alcohol is heated under reflux with an excess of the oxidizing agent. When the reaction is complete, the carboxylic acid is distilled off. The full equation for the oxidation of ethanol to ethanoic acid is:
\[ 3CH_3CH_2OH + 2Cr_2O_7^{2-} + 16H+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O \tag{17.7.1}\]
The more usual simplified version looks like this:
\[ CH_3CH_2OH + 2[O] \rightarrow CH_3COOH + H_2O \tag{17.7.2}\]
Alternatively, you could write separate equations for the two stages of the reaction - the formation of ethanal and then its subsequent oxidation.
\[ CH_3CH_2OH + [O] \rightarrow CH_3CHO + H_2O \tag{17.7.3}\]
\[ CH_3CHO + [O] \rightarrow CH_3COOH \tag{17.7.4}\]
This is what is happening in the second stage:

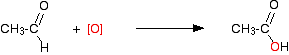
Secondary alcohols
Secondary alcohols are oxidized to ketones - and that's it. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate(VI) solution acidified with dilute sulfuric acid, you get propanone formed. Playing around with the reaction conditions makes no difference whatsoever to the product. Using the simple version of the equation and showing the relationship between the structures:

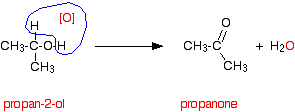
If you look back at the second stage of the primary alcohol reaction, you will see that an oxygen "slotted in" between the carbon and the hydrogen in the aldehyde group to produce the carboxylic acid. In this case, there is no such hydrogen - and the reaction has nowhere further to go.
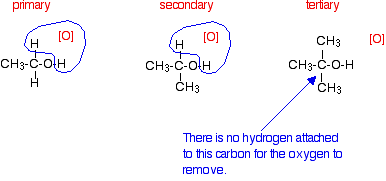
Tertiary alcohols
Tertiary alcohols are not oxidized by acidified sodium or potassium dichromate(VI) solution - there is no reaction whatsoever. If you look at what is happening with primary and secondary alcohols, you will see that the oxidizing agent is removing the hydrogen from the -OH group, and a hydrogen from the carbon atom attached to the -OH. Tertiary alcohols don't have a hydrogen atom attached to that carbon.
You need to be able to remove those two particular hydrogen atoms in order to set up the carbon-oxygen double bond.
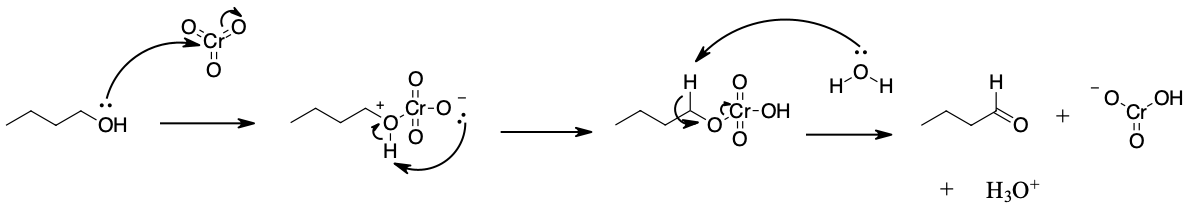
Mechanism
During this reaction mechanism the chromium atom is being reduced from Cr(VI) in the CrO3 starting material to Cr(IV) in the H2CrO3 product. Also, notice the the C=O bond is formed in the third step of the mechanism through an E2 reaction. Although E2 reaction are generally know for forming C=C double bonds thought the elimination of a halide leaving group, in this case they are use to generate a C=O through the elimination of a reduced metal as a leaving group.
Examples
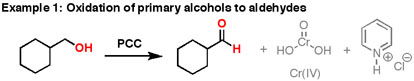
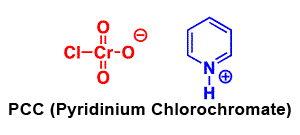
Formation of Aldehydes using PCC
Pyridinium chlorochromate (PCC) is a milder version of chromic acid.
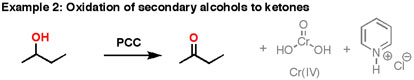
PCC oxidizes alcohols one rung up the oxidation ladder, from primary alcohols to aldehydes and from secondary alcohols to ketones. Unlike chromic acid, PCC will not oxidize aldehydes to carboxylic acids. Similar to or the same as: \(CrO_3\) and pyridine (the Collins reagent) will also oxidize primary alcohols to aldehydes. Here are two examples of PCC in action.
- If you add one equivalent of PCC to either of these alcohols, you obtain the oxidized version. The byproducts (featured in grey) are Cr(IV) as well as pyridinium hydrochloride.
- One has to be careful with the amount of water present in the reaction. If water were present, it can ad to the aldehyde to make the hydrate, which could be further oxidized by a second equivalent of PCC were it present. This is not a concern with ketones, since there is no H directly bonded to C.
How does it work? Oxidation reactions of this sort are actually a kind of elimination reaction. We’re going from a carbon-oxygen single bond to a carbon-oxygen double bond. The elimination reaction can occur because we’re putting a good leaving group on the oxygen, namely the chromium, which will be displaced when the neighboring C-H bond is broken with a base.
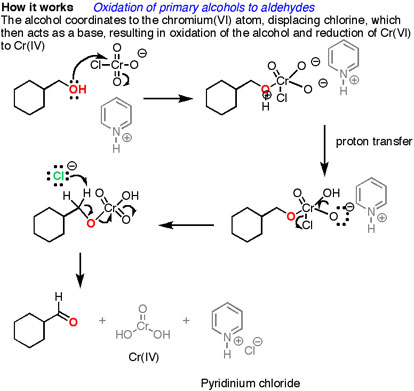
The first step is attack of oxygen on the chromium to form the Cr-O bond. Secondly, a proton on the (now positive) OH is transferred to one of the oxygens of the chromium, possibly through the intermediacy of the pyridinium salt. A chloride ion is then displaced, in a reaction reminiscent of a 1,2 elimination reaction, to form what is known as a chromate ester.
The C-O double bond is formed when a base removes the proton on the carbon adjacent to the oxygen. [aside: I've drawn the base as Cl(-) although there are certainly other species which could also act as bases here (such as an alcohol). It is also possible for pyridine to be used as the base here, although only very low concentrations of the deprotonated form will be present under these acidic conditions.] The electrons from the C-H bond move to form the C-O bond, and in the process break the O-Cr bond, and Cr(VI) becomes Cr(IV) in the process (drawn here as O=Cr(OH)2 ).
Real life notes: If you end up using PCC in the lab, don’t forget to add molecular sieves or Celite or some other solid to the bottom of the flask, because otherwise you get a nasty brown tar that is a real major pain to clean up. The toxicity and mess associated with chromium has spurred the development of other alternatives like TPAP, IBX, DMP, and a host of other neat reagents you generally don’t learn about until grad school.
Oxidation of 1o Alcohols with Dess‑Martin Periodinane (DMP) to form Aldehydes
PCC is being replaced in laboratories by Dess‑Martin periodinane (DMP) in dichloromethane solvent, which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous conditions (lower reaction temperature and a nonacidic medium). DMP is named after Daniel Dess and James Martin, who developed it in 1983.
Dess‑Martin periodinane (DMP)
General Reactions
Oxidation of Cyclohexanol to Cyclohexanone
Oxidation of Benzyl Alcohol to Benzaldehyde
Mechanism
The first step of the mechanism involves the reactant alcohol attacking the Iodine (V) atom and eliminating an acetate (Ac-) leaving group to form a periodinate intermediate. The next step is a concerted E2-like reaction where a hydrogen is removed from the alcohol, the C=O bond is formed, an acetate group is eliminated from the iodine atom, and the iodine (V) atom gains two electrons to be reduced to iodine (III).
Exercises
Draw the alcohol that the following ketones/aldehydes would have resulted from if oxidized. What oxidant could be used?
- Answer
- a) Any oxidant capable of oxidizing an alcohol to a ketone would work, such as the Jones reagent (CrO3, H2SO4, H2O), PCC, or Dess-Martin periodinane.
b) Since this is a primary alcohol, there are some precautions necessary to avoid formation of the carboxyllic acid. Milder oxidants such as the Dess-Martin periodinane, and also PCC (there is no water to form the carboxyllic acid) would work
c) Any oxidant capable of oxidizing an alcohol to a ketone would work, such as the Jones reagent (CrO3, H2SO4, H2O), PCC, or Dess-Martin periodinane.
Show the products of the oxidation of 1-propanol and 2-propanol with chromic acid in aqueous solution.
- Answer
-
Show the products of the oxidation of 1-propanol and 2-propanol with Dess-Martin periodinane.
- Answer
-
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)
James Ashenhurst (MasterOrganicChemistry.com)