7.11: Evidence for the Mechanism of Electrophilic Additions- Carbocation Rearrangements
- Page ID
- 67132
After completing this section, you should be able to explain the “unusual” products formed in certain reactions in terms of the rearrangement of an intermediate carbocation.
Make certain that you can define, and use in context, the key terms below.
- alkyl shift
- hydride shift
Whenever possible, carbocations will rearrange from a less stable isomer to a more stable isomer. This rearrangement can be achieved by either a hydride shift, where a hydrogen atom migrates from one carbon atom to the next, taking a pair of electrons with it; or an alkyl shift, in which an alkyl group undergoes a similar migration, again taking a bonding pair of electrons with it. These migrations usually occur between neighbouring carbon atoms, and hence are termed 1,2-hydride shifts or 1,2-alkyl shifts.
[A hydride ion consists of a proton and two electrons, that is, [H:]−. Hydride ions exist in compounds such as sodium hydride, NaH, and calcium hydride, CaH2.]
An electrophilic reaction such as HX with an alkene will often yield more than one product. This is strong evidence that the mechanism includes intermediate rearrangement steps of the cation.
1,2-Hydride Shift
A 1,2-hydride shift is a carbocation rearrangement in which a hydrogen atom in a carbocation migrates to the carbon atom bearing the formal charge of +1 (carbon 2) from an adjacent carbon (carbon 1).
eg:

see also 1,2-aryl shift
1,2-Alkyl Shift
A 1,2-alkyl shift is a carbocation rearrangement in which an alkyl group migrates to the carbon atom bearing the formal charge of +1 (carbon 2) from an adjacent carbon atom (carbon 1), e.g.
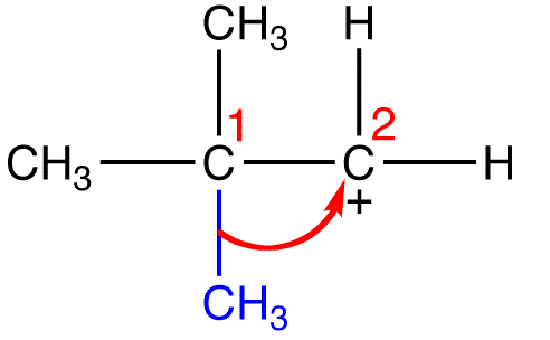
see also 1,2-aryl shift, hydride shift, alkyl shift
Exercises
Draw the expectred produts of the following reaction.
- Answer
-
The second phase of the cyclase reaction mechanism involves multiple rearrangement steps and a deprotonation. Please supply the missing mechanistic arrows.
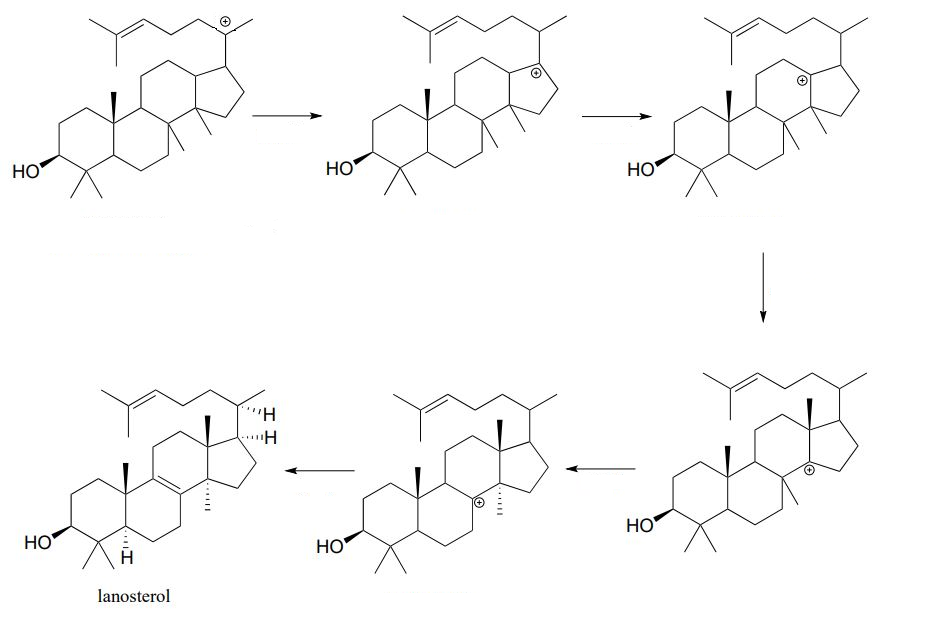
- Answer
-
The following reaction shows a rearrangement within the mechanism. Propose a mechanism that shows this.
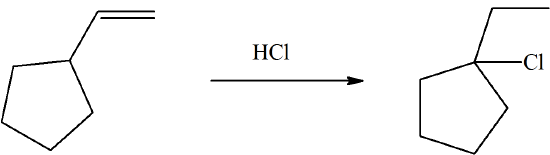
- Answer
-
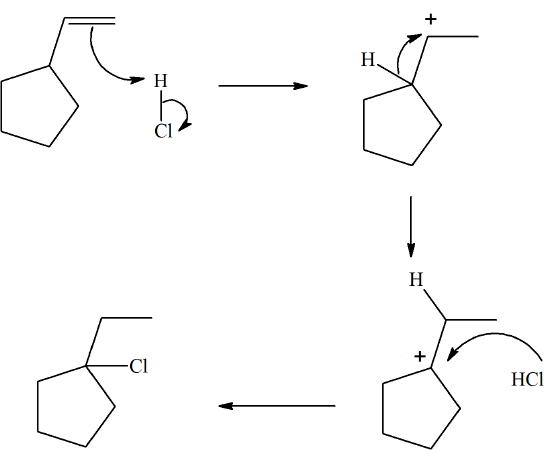
Propose a mechanism for the following reaction. It involves an electrophilic addition and the shift of a C-C and a C-H bond.
- Answer
-
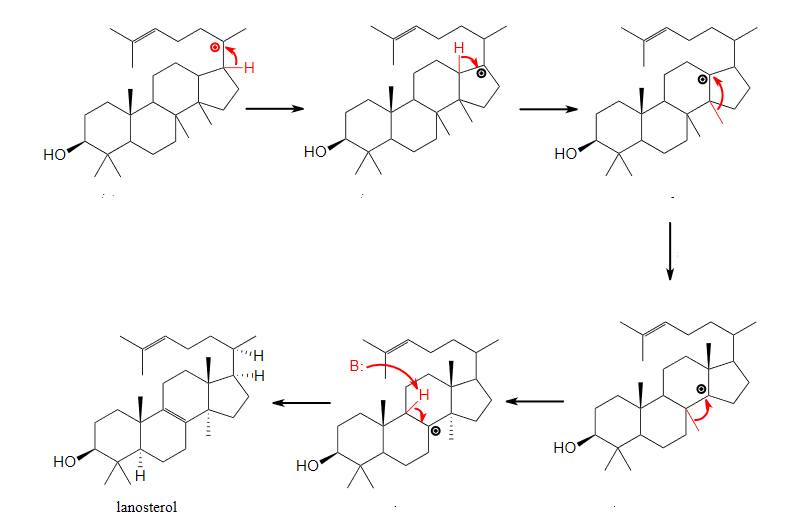
In most examples of carbocation rearrangements that you are likely to encounter, the shifting species is a hydride or methyl group. However, pretty much any alkyl group is capable of shifting. Sometimes, the entire side of a ring will shift over in a ring-expanding rearrangement.
The first 1,2-alkyl shift is driven by the expansion of a five-membered ring to a six-membered ring, which has slightly less ring strain. A hydride shift then converts a secondary carbocation to a tertiary carbocation, which is the electrophile ultimately attacked by the bromide nucleophile.
Contributors
- Gamini Gunawardena from the OChemPal site (Utah Valley University)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)

