10.7: Organometallic Coupling Reactions
- Page ID
- 67257
Objectives
After completing this section, you should be able to
- write an equation for the formation of an alkyllithium from an alkyl halide.
- write an equation for the formation of a lithium dialkylcopper (Gilman) reagent from an alkyllithium and copper(I) iodide.
- write an equation for the coupling of a lithium dialkylcopper reagent with an alkyl halide (i.e., a Corey-House synthesis).
- draw the structure of the product formed from a given Corey-House synthesis.
- identify the reagents needed to convert two given organohalides to a specified hydrocarbon through a Corey-House synthesis.
Make certain that you can define, and use in context, the key terms below.
- Corey-House synthesis
- pheromone
A pheromone is a chemical released by members of one species to cause specific behavioural or physiological changes in other members of the same species. Examples include sex pheromones, alarm pheromones and trail pheromones.
The Corey-House synthesis provides us with a method of coupling together two alkyl groups through the formation of a new carbon-carbon bond. The product of such a reaction is an alkane, and this synthetic method gives us a route for the preparation of unsymmetrical alkanes. The method was developed during the late 1960s by E. J. Corey and Herbert House working independently at Harvard University and Massachusetts Institute of Technology, respectively. The overall synthetic route is shown on the next page. Note that R and R′ represent alkyl groups, which can be the same or different, and X represents a halogen (preferably bromine or iodine).
In order to obtain a good yield of alkane, both R′X and RX should be primary alkyl halides. However, the experimental procedure can be modified so that this synthesis can be carried out using a wide range of alkyl, aryl, vinyl, benzyl and allyl halides. A detailed discussion of these modifications is beyond the scope of this course, but you should be aware of possible limitations in the use of the Corey-House synthesis.
Note: In some textbooks, lithium diorganocoppers are referred to as lithium dialkylcoppers, or cuprates.
Gilman Reagents
Another important reaction exhibited by organometallic reagents is metal exchange. Organolithium reagents react with cuprous iodide to give a lithium dimethylcopper reagent, which is referred to as a Gilman reagent. Gilman reagents are a source of carbanion like nucleophiles similar to Grignard and Organo lithium reagents. However, the reactivity of organocuprate reagents is slightly different and this difference will be exploited in different situations. In the case of α, β unsaturated carbonyls organocuprate reagents allow for an 1,4 addition of an alkyl group. As we will see later Grignard and Organolithium reagents add alkyl groups 1,2 to α, β unsaturated carbonyls
Organocuprate reagents are made from the reaction of organolithium reagents and \(CuI\)
\[ 2RLi + CuI \rightarrow R_2CuLi + LiI\]
This acts as a source of R:-
\[2 Ch_3Li + CuI \rightarrow (CH_3)_2CuLi + LiI\]
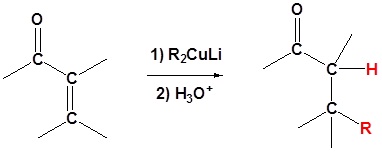
Example
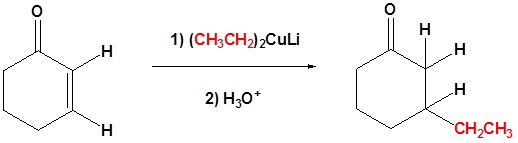
Coupling Reactions with Gilman Reagents
The coupling of Giman reactions with organochlorides, organobromides, and organoiodides is useful in organic synthesis because it forms a carbon bond. During the reaction one of the alkyl groups fromt he Gilman reagent replaces the halogen for the organohalide.
Examples
Exercise
- Starting with alkyl halides containing no more than four carbon atoms, how would you synthesize each of the following alkanes?
- 2,5-dimethylhexane
- 2-methylhexane
- Answer
-
Notice that in (a), both the alkyl halides are primary. This fact should ensure a good yield of product. In (b) we have the choice of using 2-bromopropane and 1-bromobutane, or 1-bromo-2-methylpropane and 1-bromopropane. We chose the latter as it enables us to use two primary alkyl halides, and hence a simpler procedure
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

