7.6: Stability of Alkenes
- Page ID
- 67127
After completing this section, you should be able to
- explain why cis alkenes are generally less stable than their trans isomers.
- explain that catalytic reduction of a cis alkene produces the same alkane as the catalytic reduction of the trans isomer.
- explain how heats of hydrogenation (ΔH°hydrog) can be used to show that cis alkenes are less stable than their trans isomers, and discuss, briefly, the limitations of this approach.
- arrange a series of alkenes in order of increasing or decreasing stability.
- describe, briefly, two of the hypotheses proposed to explain why alkene stability increases with increased substitution. [Note: This problem is a typical example of those instances in science where there is probably no single “correct” explanation for an observed phenomenon.]
Make certain that you can define, and use in context, the key terms below.
- catalytic hydrogenation
- heat of hydrogenation, (ΔH°hydrog)
- hyperconjugation
The two alkenes, cis $\ce{\sf{CH3CH=CHCH3}}$ and $\ce{\sf{(CH3)2C=CH2}}$ have similar heats of hydrogenation (−120 kJ/mol and −119 kJ/mol, respectively), and are therefore of similar stability. However, they are both less stable than trans $\ce{\sf{CH3CH=CHCH3}}$ (−116 kJ/mol).
You may wonder why an sp2 -sp3 bond is stronger than an sp3-sp3 bond. Bond strength depends on the efficiency with which orbitals can overlap. In general, s orbitals overlap more efficiently than do p orbitals; therefore, the s-s bond in the hydrogen molecule is stronger than the p-p bond in fluorine. In hybrid orbitals, the greater the s character of the orbital, the more efficiently it can overlap: an sp2 orbital, which has a 33% s character, can overlap more effectively than an sp3 orbital, with only 25% s character.
Alkene hydrogenation is the syn-addition of hydrogen to an alkene, saturating the bond. The alkene reacts with hydrogen gas in the presence of a metal catalyst which allows the reaction to occur quickly. The energy released in this process, called the heat of hydrogenation, indicates the relative stability of the double bond in the molecule (see Catalytic Hydrogenation).
Introduction
The reaction begins with H2 gas and an alkene (a carbon-carbon double bond). The pi bond in the alkene acts as a nucleophile; the two electrons in it form a sigma bond with one of the hydrogen atoms in H2. With the pi bond broken, the other carbon (the one that did not newly receive a hydrogen) is left with a positive formal charge. This is the carbocation intermediate. The remaining (unreacted) hydrogen is now a hydride anion, as it was left with two electrons previously in the H-H sigma bond. Next, the electrons of the negatively charged hydride ion form a bond with the positively charged carbon. This reaction is exothermic. It will occur, but it is very slow without a catalyst.
The Catalyst
A catalyst increases the reaction rate by lowering the activation energy of the reaction. Although the catalyst is not consumed in the reaction, it is required to accelerate the reaction sufficiently to be observed in a reasonable amount of time. Catalysts commonly used in alkene hydrogenation are: platinum, palladium, and nickel. The metal catalyst acts as a surface on which the reaction takes place. This increases the rate by putting the reactants in close proximity to each other, facilitating interactions between them. With this catalyst present, the sigma bond of H2 breaks, and the two hydrogen atoms instead bind to the metal (see #2 in the figure below). The \(\pi\) bond of the alkene weakens as it also interacts with the metal (see #3 below).
Since both the reactants are bound to the metal catalyst, the hydrogen atoms can easily add, one at a time, to the previously double-bonded carbons (see #4 and #5 below). The position of both of the reactants bound to the catalyst makes it so the hydrogen atoms are only exposed to one side of the alkene. This explains why the hydrogen atoms add to same side of the molecule, called syn-addition.
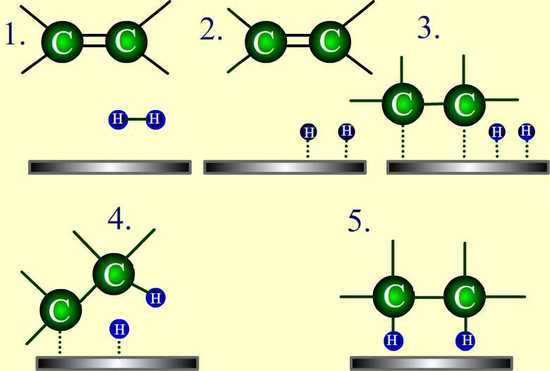
Figure 7.6.1: Hydrogenation takes place in the presence of a metal catalyst.
The catalyst remains intact and unchanged throughout the reaction.
Heats of Hydrogenation
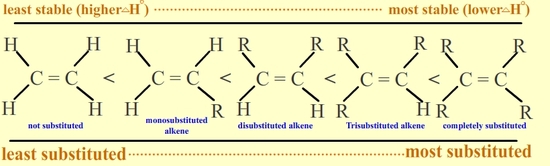
The stability of alkene can be determined by measuring the amount of energy associated with the hydrogenation of the molecule. Since the double bond is breaking in this reaction, the energy released in hydrogenation is proportional to the energy in the double bond of the molecule. This is a useful tool because heats of hydrogenation can be measured very accurately. The \(\Delta H^o\) is usually around -30 kcal/mol for alkenes. Stability is simply a measure of energy. Lower energy molecules are more stable than higher energy molecules. More substituted alkenes are more stable than less substituted ones due to hyperconjugation. They have a lower heat of hydrogenation. The following illustrates stability of alkenes with various substituents:
In disubstituted alkenes, trans isomers are more stable than cis isomers due to steric hindrance. Also, internal alkenes are more stable than terminal ones. See the following isomers of butene:
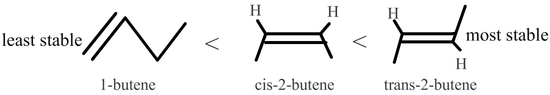
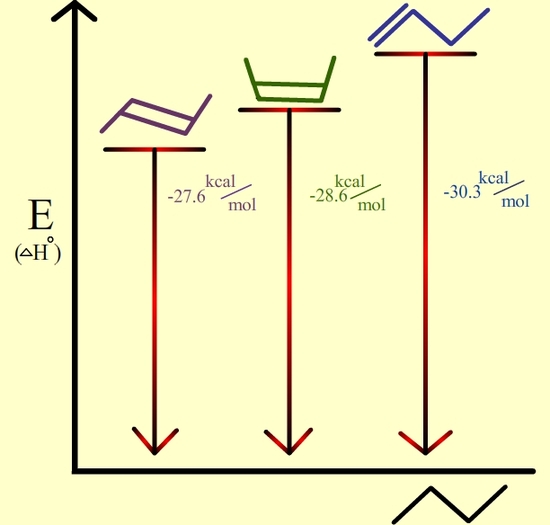
Figure 7.6.3: Trans-2-butene is the most stable because it has the lowest heat of hydrogenation.
In cycloalkenes smaller than cyclooctene, the cis isomers are more stable than the trans as a result of ring strain.
Outside Links
- Helpful Information: http://www.wou.edu/las/physci/ch334/...ure/lect16.htm
- Hydrogenation Wikipedia page: http://en.Wikipedia.org/wiki/Hydrogenation
- More professional animation: http://www.jbpub.com/organic-online/movies/cathyd.htm
References
- Fox, Marye Anne, and James K. Whitesell. Organic Chemistry. 3rd ed. Sudbury, MA: Janes and Bartlett Publishers, 2004.
- Hanson, James R. Functional Group Chemistry. Bristol, UK: The Royal Society of Chemistry, 2001.
- Streitwieser, Andrew Jr., and Clayton H. Heathcock. Introduction to Organic Chemistry. 2nd ed. New York, NY: Macmillan Publishing Co., Inc., 1981.
- Vollhardt, Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. 5th ed. New York, NY: W.H. Freeman and Company, 2007.
- Zlatkis, Albert, Eberhard Breitmaier, and Gunther Jung. A Concise Introduction to Organic Chemistry. New York: McGraw-Hill Book Company, 1973.
Exercises
1) Of the three following isomers which would be expected to be the most stable?
a)
b)
c)
- Answer
-
1)
a)
b)
c)
3-Bromobut-1-ene reacts with hydrogen gas in the presence of a platinum catalyst. What is the name of the product?
- Answer
-
2-Bromobutane (numbering changes when alkene is no longer present)
Cyclohexene reacts with hydrogen gas in the presence of a palladium catalyst. What is the name of the product?
- Answer
-
Cyclohexane
What is the stereochemistry (syn or anti addition) of an alkene hydrogenation reaction?
- Answer
-
Syn-addition
When looking at their heats of hydrogenation, is the cis or the trans isomer generally more stable?
- Answer
-
Trans
Show the product for the following
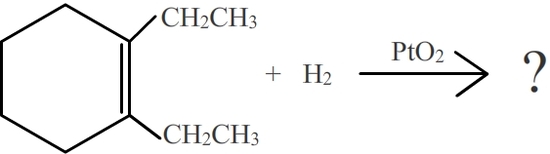
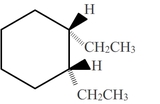
- Answer
-
Contributors and Attributions
- Anna Manis (UCD)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

