22.4: 22.3a Carbonyl Condensations - The Aldol Reaction
- Page ID
- 148490
Objectives
After completing this section, you should be able to
- write a general mechanism for carbonyl condensation reactions.
- write an equation to illustrate the aldol condensation reaction.
- identify the product formed when an aldehyde or ketone having an alpha‑hydrogen atom is treated with base in a protic medium.
- identify the aldehyde or ketone, and other reagents required to produce a given β‑hydroxy carbonyl compound by an aldol reaction.
- determine whether a given aldehyde or ketone will undergo an aldol reaction.
- write the detailed mechanism of the aldol reaction.
Key Terms
- aldol
- aldol reaction
- carbonyl condensation reaction (see Chapter 18 Affix)
Study Notes
It is important that you understand the general mechanism of carbonyl condensation described in this section: once you grasp this mechanism, you will see that all the reactions that follow are very similar.
The aldol reaction is sometimes referred to as the aldol condensation. However, a condensation reaction is often regarded as a reaction in which two molecules join together with the elimination of a molecule of water (or some other compound of low molar mass). Thus, the aldol reaction described here is not a true condensation; the true aldol condensation is described later, in Section 23.3. It is perhaps unfortunate that the reactions discussed in this unit are all described as condensation reactions whether or not water is eliminated.
The term “aldol” (from "aldehyde alcohol") is used both to describe the specific compound 3‑hydroxybutanal:

and to describe β‑hydroxy aldehydes in general.
A useful carbon-carbon bond-forming reaction known as the Aldol Reaction is yet another example of electrophilic substitution at the alpha carbon in enolate anions. The fundamental transformation in this reaction is a dimerization of an aldehyde (or ketone) to a beta-hydroxy aldehyde (or ketone) by alpha C–H addition of one reactant molecule to the carbonyl group of a second reactant molecule. Due to the carbanion like nature of enolates they can add to carbonyls in a similar manner as Grignard reagents. For this reaction to occur at least one of the reactants must have α hydrogens.
General Aldol reaction
Going from reactants to products simply
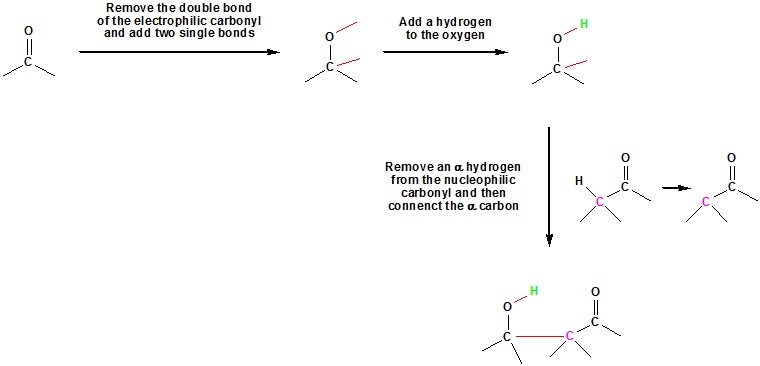
Example 23.1.1: Aldol Reactions
Aldol Reaction Mechanism
Step 1: Enolate formation
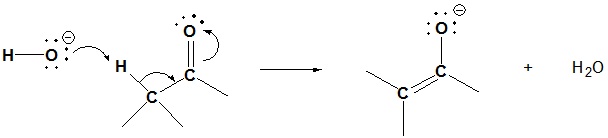
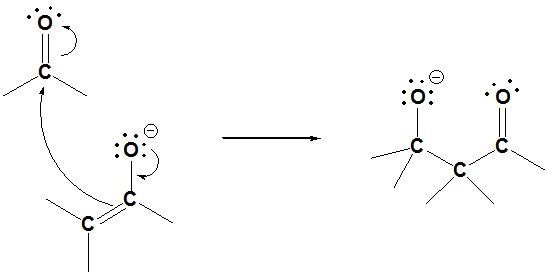
Step 2: Nucleophilic attack by the enolate
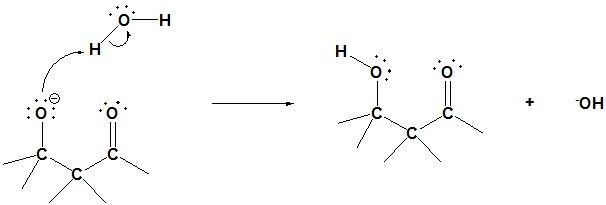
Step 3: Protonation
OBJECTIVES
After completing this section, you should be able to describe the difference between a carbonyl condensation reaction and an alpha‑substitution reaction, and determine which of these two types of reaction is most likely to occur, given the appropriate experimental data.
STUDY NOTES
So far we have discussed three of the four general reactions of carbonyl compounds: nucleophile additions of aldehydes and ketones (Chapter 19), nucleophilic acyl substitution reactions of carboxylic acid derivatives (Chapter 21) and alpha‑substitution reactions (Chapter 22). The fourth general reaction, carbonyl condensation, is similar to the alpha‑substitution reaction, so you need to appreciate how it differs from the other three and the conditions under which it occurs.
Carbonyl condensation reactions are a type of alpha-substitution reaction. Both occur through an enolate ion intermediate under basic conditions and involve substitution at the carbon alpha to the carbonyl group. However, in a carbonyl condensation reaction the electrophile (E+) being attacked is another carbonyl compound.
In a carbonyl condensation a catalytic amount of base is used to generate the enolate ion which attacks any unreacted carbonyl compound to form the carbon-carbon bond at the alpha site. The resulting alkoxide is then protonated, which regenerates the base that will produce more enolate ion in the next cycle.
These steps are all reversible and it should be noted that reactants and products that are close in energy level can potentially undergo the reverse reaction if conditions change enough. While from a synthetic point of view in the laboratory this may mean increasing yields by driving the reaction to completion (e.g. adding heat, removing product), in biological systems it can have more drastic consequences. Indeed, depending on metabolic conditions, retro-aldol reactions (the reverse of aldol condensations, in which carbon-carbon bonds are broken) can occur.
In contrast, the alpha-substitution reaction is often more directional by design. To reduce unwanted competition from carbonyl condensation, the enolate ion intermediate is generated all at once with a full equivalent of strong base at low temperature. The reactive enolate intermediate generated is then quenched with rapid addition of the electrophile to complete the substitution. In Section 22.7, under direct alkylation, the use of strong bases like NaNH2 and LDA to generate the enolate intermediate followed by addition of an alkylhalide was discussed.
EXAMPLE 22.7.1: ALPHA ALKYLATION
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

