22.2: 22.1 Intro to Enols and Enolates
- Page ID
- 148252
Objectives
After completing this section, you should be able to
- write an equation to illustrate keto‑enol tautomerism.
- write a detailed mechanism for acid‑catalyzed keto‑enol tautomerism.
- write a detailed mechanism for base‑catalyzed keto‑enol tautomerism.
- draw the structure of the enol form of a given carbonyl compound.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- enol
- keto
- tautomerism
- tautomers
- enolate ion
Study Notes
Keto‑enol tautomerism was first introduced in Section 9.4, in the discussion of the hydration of alkynes. The subject was raised again in the chapter entitled A Preview of Carbonyl Compounds, during the brief overview of the alpha‑substitution reactions of carbonyl compounds. You may wish to review these sections before proceeding.
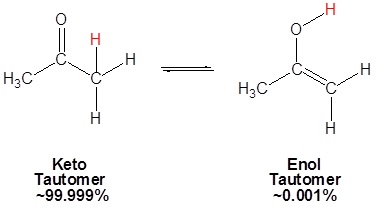
Because of the acidity of α hydrogens carbonyls undergo keto-enol tautomerism. Tautomers are rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom and a differently located double bond. The equilibrium between tautomers is not only rapid under normal conditions, but it often strongly favors one of the isomers (acetone, for example, is 99.999% keto tautomer). Even in such one-sided equilibria, evidence for the presence of the minor tautomer comes from the chemical behavior of the compound. Tautomeric equilibria are catalyzed by traces of acids or bases that are generally present in most chemical samples.
Mechanism for Enol Formation
Acid conditions
1) Protonation of the Carbonyl
2) Enol formation
Basic conditions
1) Enolate formation
2) Protonation
How Enols React
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

