21.17: 21.8 Nucleophilic Acyl Substitution Reactions of Carboxylic Acids
- Page ID
- 144048
Objectives
After completing this section, you should be able to
- write an equation to describe the formation of
- an acid chloride from a carboxylic acid.
- an ester from a carboxylic acid.
- an amide from a carboxylic acid.
- an alcohol from carboxylic acid.
- write an equation to represent the formation of a cyclic anhydride from a dicarboxylic acid.
- write the detailed mechanism for the reaction of a carboxylic acid with thionyl chloride.
- identify the reagents necessary to convert a given carboxylic acid to a given acid chloride, ester, amide or alcohol.
- identify the carboxylic acid required for a direct synthesis of a given acid chloride, amide, ester or alcohol.
- write an equation to describe the formation of an ester through the nucleophilic attack of a carboxylate anion on an alkyl halide.
- write a detailed mechanism for the Fischer esterification reaction.
- describe the evidence from isotopic labelling experiments that is commonly cited to support the generally accepted mechanism of the Fischer esterification reaction.
- explain why the direct conversion of a carboxylic acid to an amide can only be achieved with difficulty and at a high temperature.
Key Terms
Make certain that you can define, and use in context, the key term below.
- Fischer esterfication reaction
Study Notes
If necessary, review the SN2 reaction between a carboxylate anion and a primary alkyl halide (Sections 11.2 and 11.3).
You should recall that the oxygen‑18 isotope, 18O, has a nucleus containing eight protons and ten neutrons. Because it is non‑radioactive, 18O can be safely employed in the investigation of reaction mechanisms.
Acid Anhydride Formation
An acid anhydride (or just anhydride) is the product of formal condensation of two oxoacid molecules with the release of a water molecule. The most common anhydrides in organic chemistry are those derived from carboxylic acids at high temperatures to remove water.
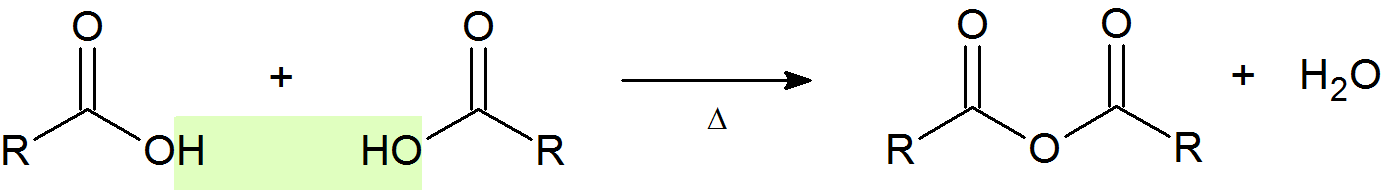
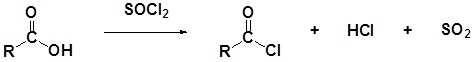
Carboxylic acids react with Thionyl Chloride (\(SOCl_2\)) to form acid chlorides
During the reaction the hydroxyl group of the carboxylic acid is converted to a chlorosulfite intermediate making it a better leaving group. The chloride anion produced during the reaction acts a nucleophile.
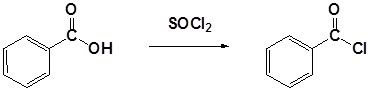
Example
Mechanism
1) Nucleophilic attack on Thionyl Chloride

2) Removal of Cl leaving group
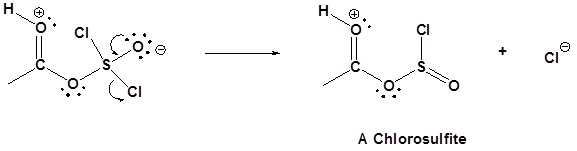
3) Nucleophilic attack on the carbonyl
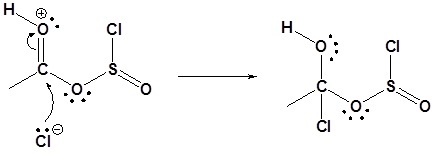
4) Leaving group removal
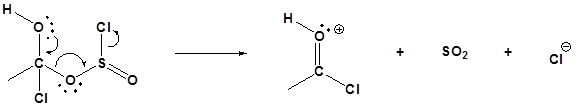
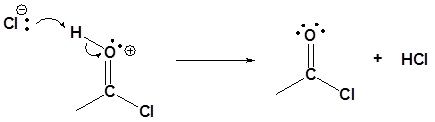
5) Deprotonation
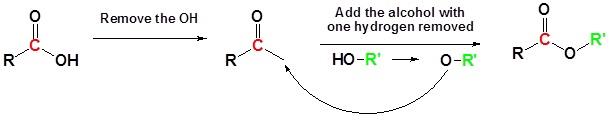
Carboxylic acids can react with alcohols to form esters in a process called Fischer esterification
Usually the alcohol is used as the reaction solvent. An acid catalyst is required.
Basic Reaction
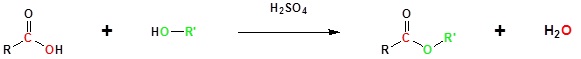
Going from reactants to products simplified
Example
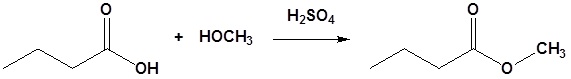
Mechanism
1) Protonation of the carbonyl by the acid. The carbonyl is now activated toward nucleophilic attack.
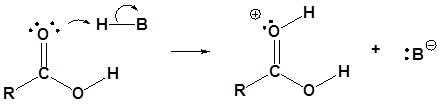
2) Nucleophilic attack on the carbonyl
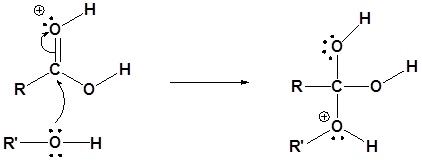
3) Proton transfer
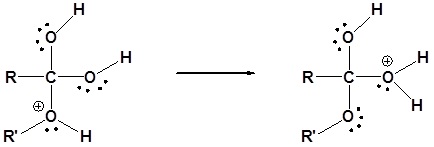
4) Water leaves
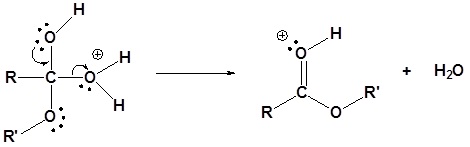
5) Deprotonation
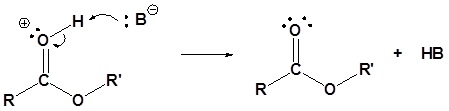
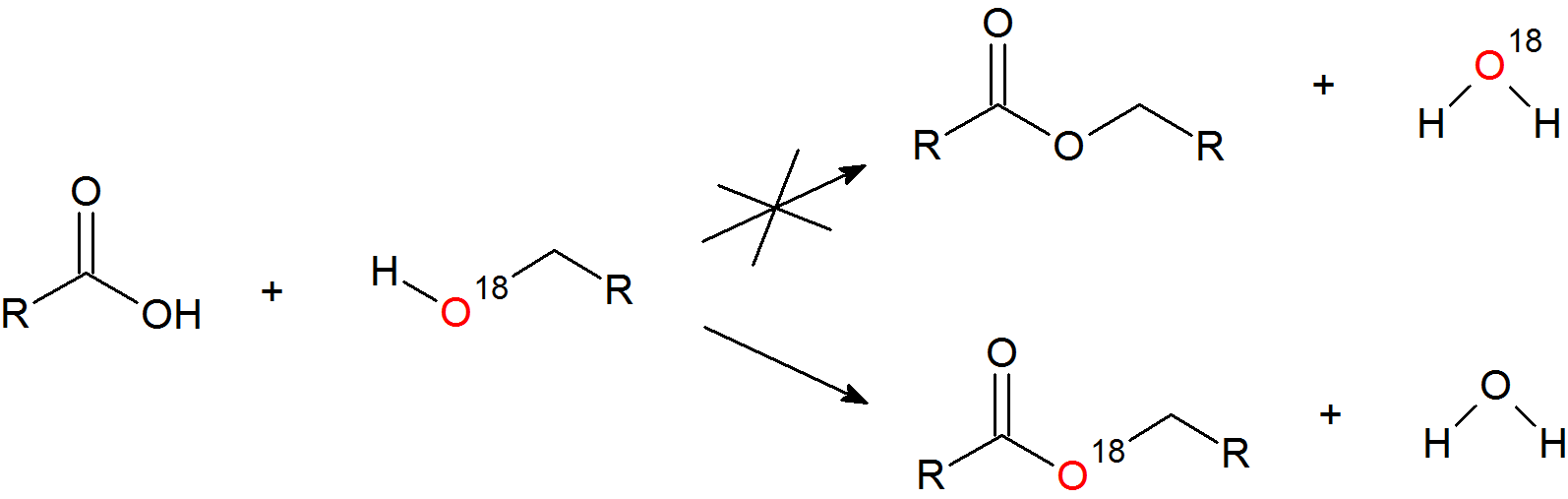
Isotopic Labeling
Evidence to support the Fischer esterfication mechanism comes from isotopic labeling experiments with oxygen-18. If the reaction is carried out with oxygen-18 labeled alcohol, the isotope is found exclusively in the ester and not the water generated.
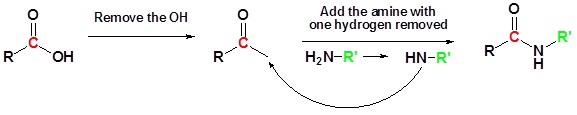
Conversion of Carboxylic Acids to Amides
The direct reaction of a carboxylic acid with an amine would be expected to be difficult because the basic amine would deprotonate the carboxylic acid to form a highly unreactive carboxylate. However when the ammonium carboxylate salt is heated to a temperature above 100 oC water is driven off and an amide is formed.
General Reaction
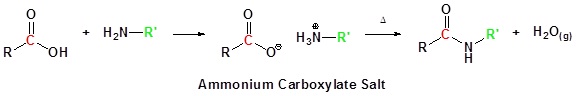
Going from reactants to products simply
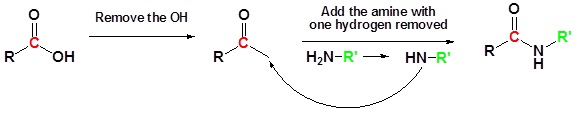
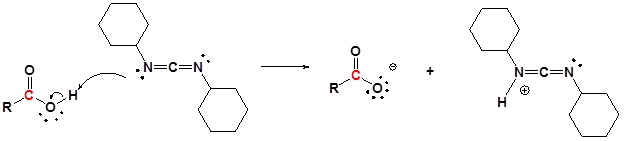
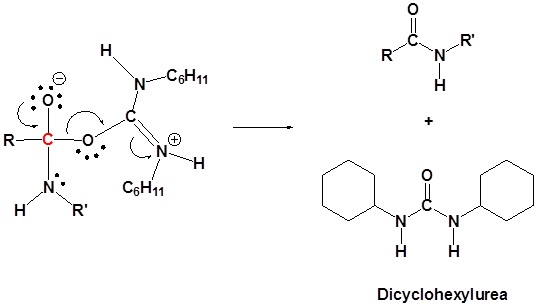
Conversion of Carboxylic acids to amide using DCC as an activating agent
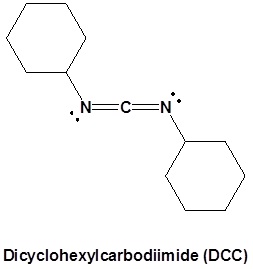
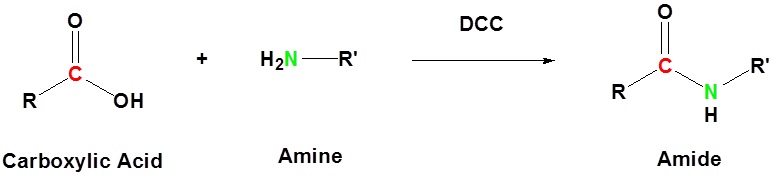
The direct conversion of a carboxylic acid to an amide is difficult because amines are basic and tend to convert carboxylic acids to their highly unreactive carboxylates. In this reaction the carboxylic acid adds to the DCC molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. DCC induced coupling to form an amide linkage is an important reaction in the synthesis of peptides.
Basic reaction
Going from reactants to products simplified
Mechanism
1) Deprotonation
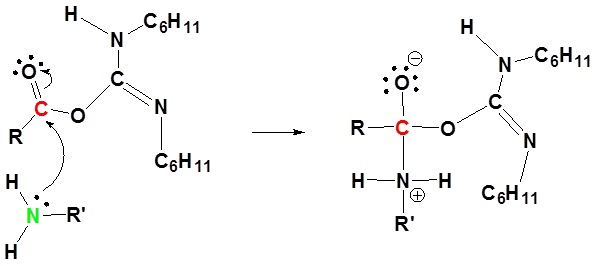
2) Nucleophilic attack by the carboxylate

3) Nucleophilic attack by the amine
4) Proton transfer
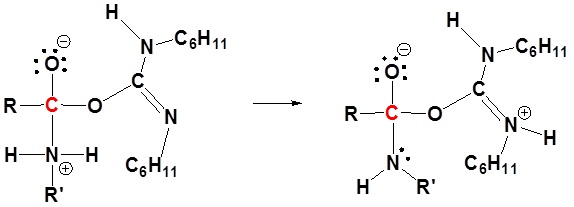
5) Leaving group removal
Carboxylic acids can be converted to 1o alcohols using Lithium aluminum hydride (LiAlH4)
Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.
General reaction
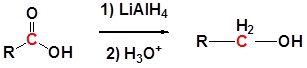
Going from reactant to products simplified
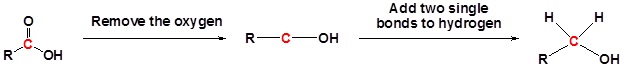
Example
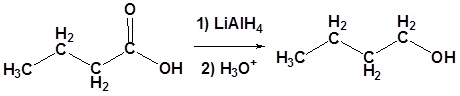
Possible Mechanism
1) Deprotonation
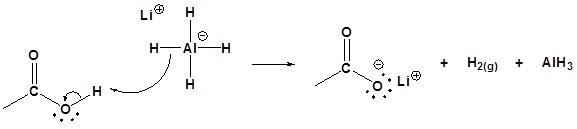
2) Nucleopilic attack by the hydride anion
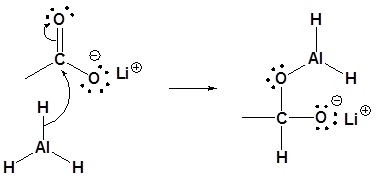
3) Leaving group removal
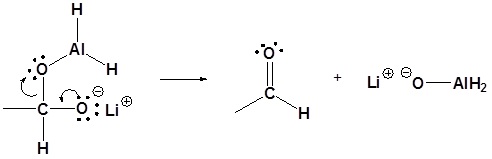
4) Nucleopilic attack by the hydride anion
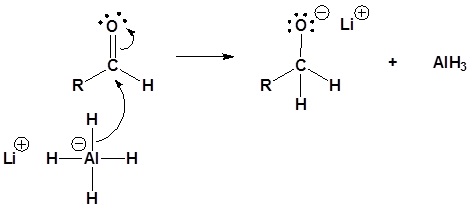
5) The alkoxide is protonated
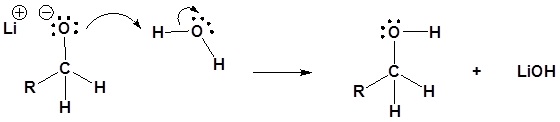
Biologic Conversion of Carboxylic Acids
In biological chemistry direct conversion of a carboxylic acid to an acyl derivative by nucleophilic acyl substitution does not occur. Rather, the first conversion is from a carboxylate (the least reactive acyl transfer substrate) to an acyl phosphate (the most reactive acyl transfer substrate). This transformation requires a reaction that we are familiar with: phosphorylation of a carboxylate oxygen with ATP as the phosphate donor.
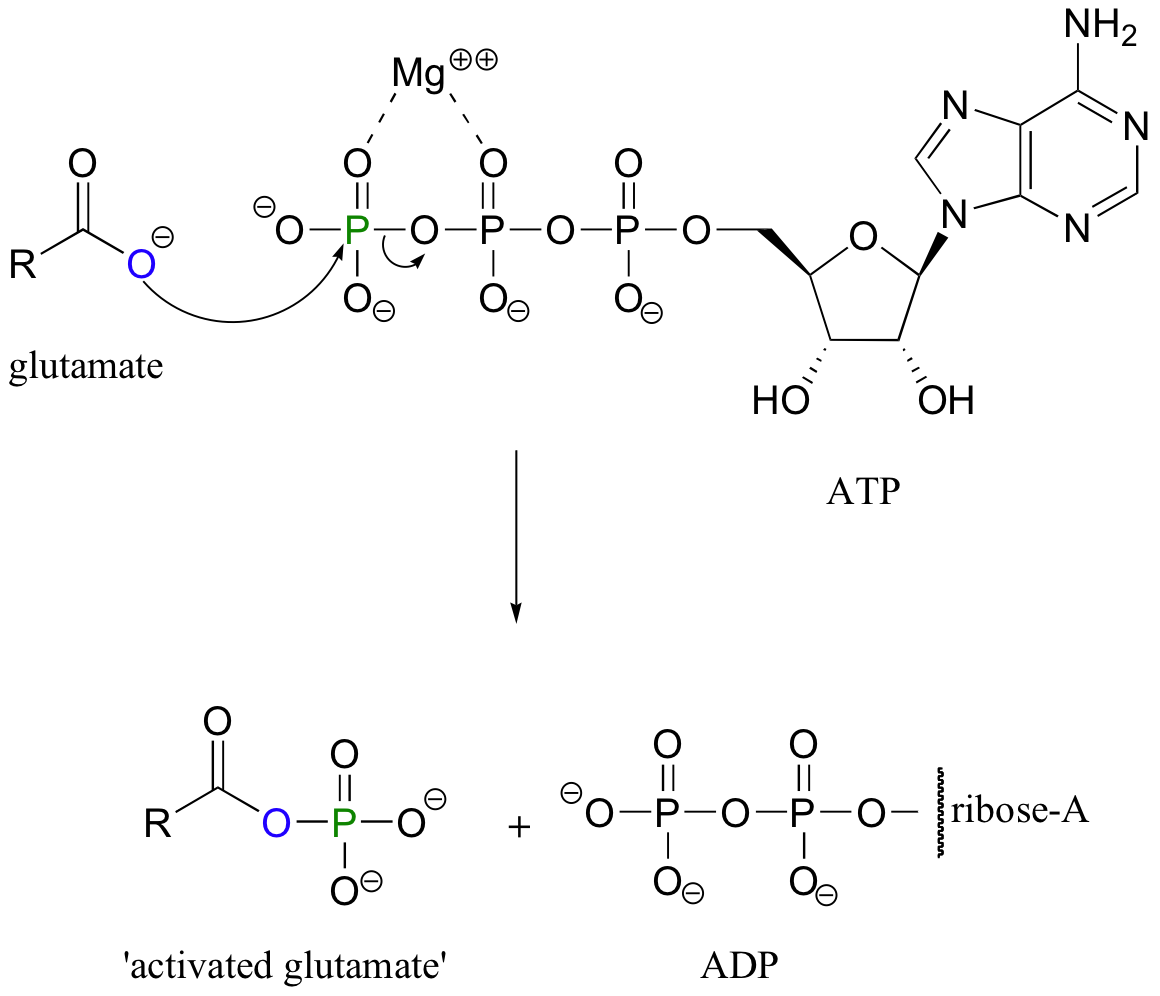
Note that this is just one of the many ways that ATP is used as a energy storage unit: in order to make a high energy acyl phosphate molecule from a low energy carboxylate, the cell must ‘spend’ the energy of one ATP molecule.
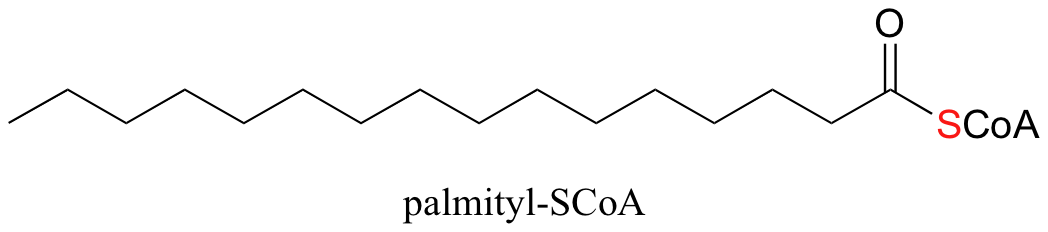
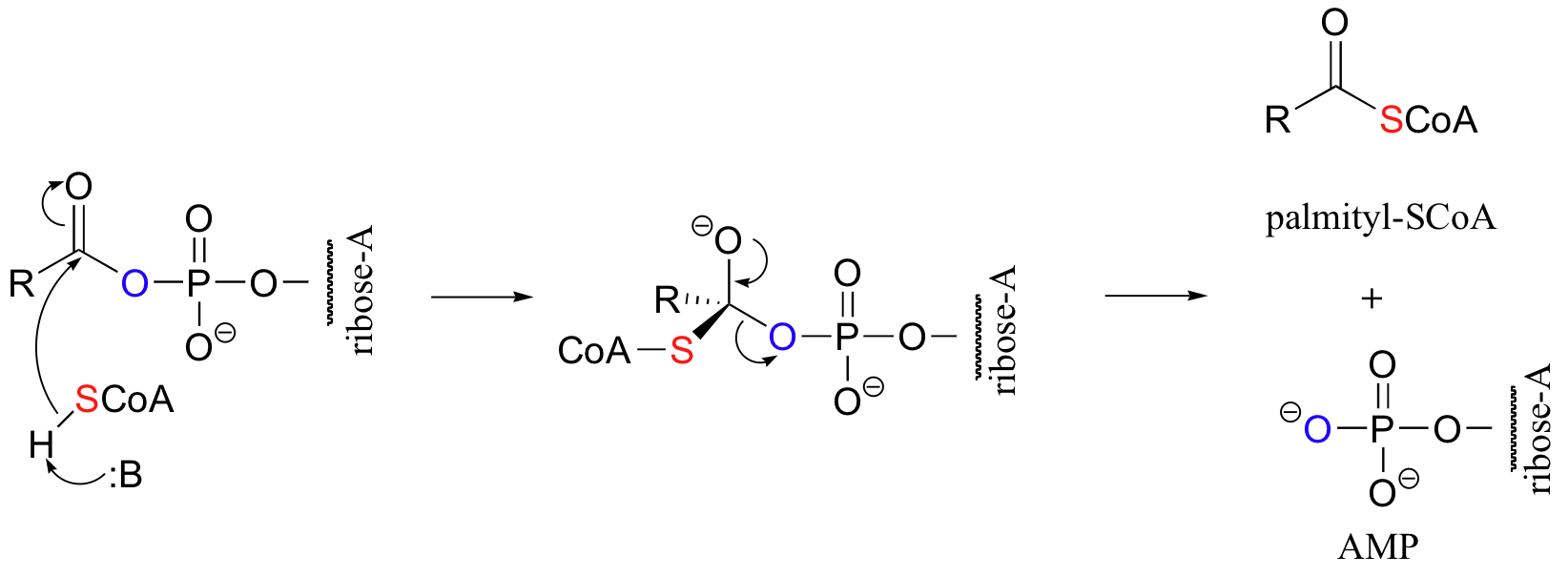
An excellent example of biological activated carboxylic acids is seed in the biosynthesis of fatty acids. In the biologically active form of fatty acids, the carboxylate groups have been converted to thioesters using coenzyme A. For example, the activated form of the C16 fatty acid palmitate is:
Let’s take a look at how this activation takes place, in a reaction catalyzed by an enzyme called acyl CoA synthetase. You already know that carboxylates are not themselves good substrates for acyl substitution reactions, and must be activated. Thus, you might predict that the first step of this reaction requires ATP to make a high-energy acyl phosphate intermediate. In fact, the activated carboxylate in this case is an acyl-AMP, formed in the same way as the acyl-AMP intermediate in the asparagine synthetase reaction (section 12.2B).
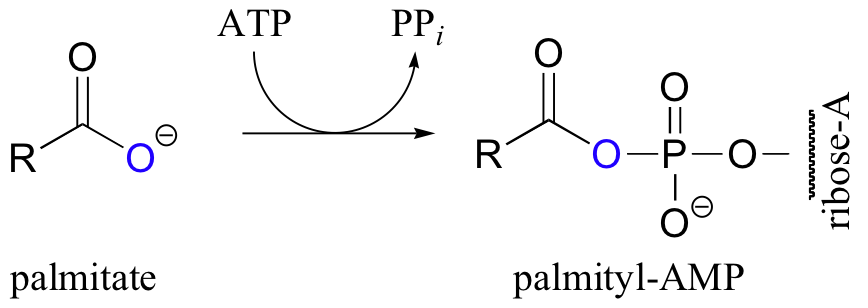
The activated acyl-AMP intermediate is then attacked by the thiol sulfur of coenzyme A, and the AMP group is expelled to form the fatty acyl CoA.
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

