2.8.2: Rules for Resonance Forms
- Page ID
- 91495
Skills to Develop
-
After completing this section, you should be able to use the concept of resonance to explain structural features of certain species; for example, why all of the carbon-oxygen bonds in the carbonate ion are the same length. This particular ion is discussed in further detail in Section 2.6.
Rules for estimating stability of resonance structures
- The greater the number of covalent bonds, the greater the stability since more atoms will have complete octets
- The structure with the least number of formal charges is more stable
- The structure with the least separation of formal charges is more stable
- A structure with a negative charge on the more electronegative atom will be more stable
- Positive charges on the least electronegative atom (most electropositive) is more stable
- Resonance forms that are equivalent have no difference in stability and contribute equally. (eg. benzene)
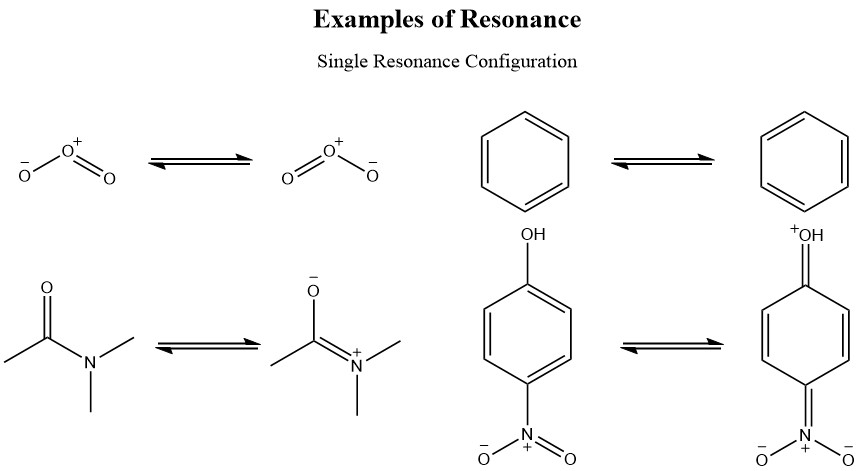
The above resonance structures show that the electrons are delocalized within the molecule and through this process the molecule gains extra stability. Ozone with both of its opposite formal charges creates a neutral molecule and through resonance it is a stable molecule. The extra electron that created the negative charge one terminal oxygen can be delocalized by resonance through the other terminal oxygen.
Benzene is an extremely stable molecule due to its geometry and molecular orbital interactions, but most importantly, due to its resonance structures. The delocalized electrons in the benzene ring make the molecule very stable and with its characteristics of a nucleophile, it will react with a strong electrophile only and after the first reactivity, the substituted benzene will depend on its resonance to direct the next position for the reaction to add a second substituent.
Example 2.5.1: Multiple Resonance of other Molecules
Molecules and ions with more than one resonance form
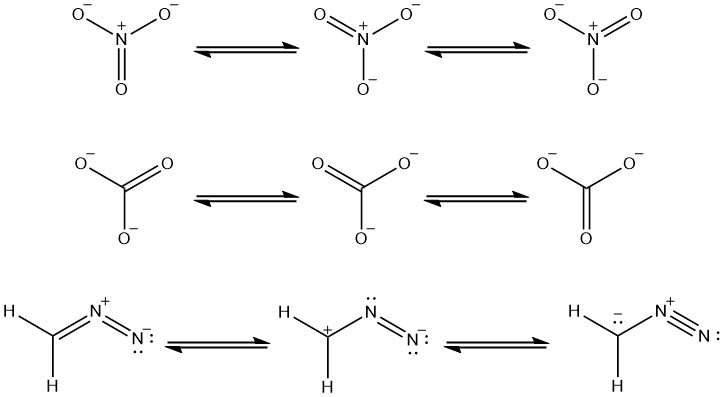
Some structural resonance conformations are the major contributor or the dominant forms that the molecule exists. For example, if we look at the above rules for estimating the stability of a molecule, we see that for the third molecule the first and second forms are the major contributors for the overall stability of the molecule. The nitrogen is more electronegative than carbon so, it can handle the negative charge more than carbon. A carbon with a negative charge is the least favorable conformation for the molecule to exist, so the last resonance form contributes very little for the stability of the Ion.
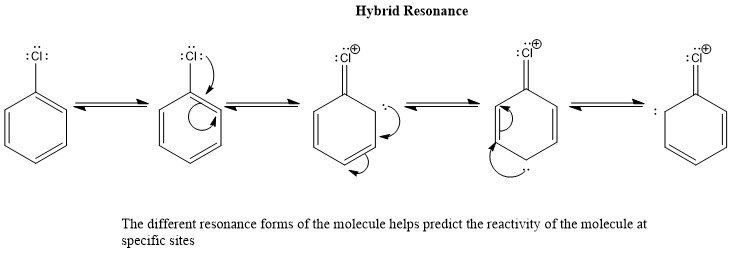
The Hybrid Resonance forms show the different Lewis structures with the electron been delocalized. This is very important for the reactivity of chloro-benzene because in the presence of an electrophile it will react and the formation of another bond will be directed and determine by resonance. The lone pair of electrons delocalized in the aromatic substituted ring is where it can potentially form a new bond with an electrophile, as it is shown there are three possible places that reactivity can take place, the first to react will take place at the para position with respect to the chloro substituent and then to either ortho position.
Exercises
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

