9.E: Advanced Theories of Covalent Bonding- Homework
- Page ID
- 360637
Turn in your answers for the following questions - show your work
- Draw Lewis dot structures, determine the hybridization of each atom and sketch the orbital structure for;
- CH4
- NH3
- HBr
- PCl5
- CO2
- BF3
- C4H8O (3 different structures)
- Draw Lewis dot structures then compare and contrast the Lewis dot, hybrid, and MO models for describing the structure of these molecules.
- H2
- O2
The Following Questions are for your practice - Do Not Turn In. They include answers so you can check your work
Valence Bond Theory
Chapter Exercises
- Draw a curve that describes the energy of a system with H and Cl atoms at varying distances. Then, find the minimum energy of this curve two ways.
- Use the bond energy found in Table 8.2.1 to calculate the energy for one single HCl bond (Hint: How many bonds are in a mole?)
- Use the enthalpy of reaction and the bond energies for \(H_2\) and \(Cl_2\) to solve for the energy of one mole of HCl bonds. \[H_{2(g)}+Cl_{2(g)} \rightleftharpoons 2HCl_{(g)} \;\;\; ΔH^∘_{rxn}=−184.7\; kJ/mol\]
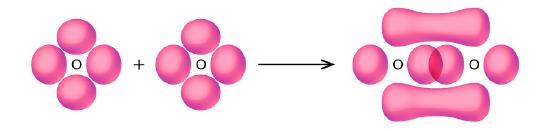
- Use valence bond theory to explain the bonding in O2. Sketch the overlap of the atomic orbitals involved in the bonds in O2.
- Draw the Lewis structures for CO2 and CO, and predict the number of σ and π bonds for each molecule.
- CO2
- CO
Solutions
1
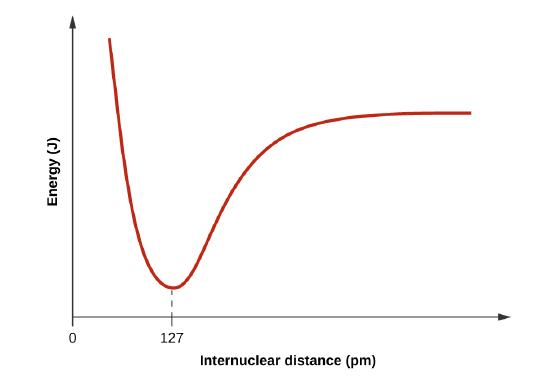
When H and Cl are separate (the x axis) the energy is at a particular value. As they approach, it decreases to a minimum at 127 pm (the bond distance), and then it increases sharply as you get closer.
2. Bonding: One σ bond and one π bond. The s orbitals are filled and do not overlap. The p orbitals overlap along the axis to form a σ bond and side by side to form the π bond.
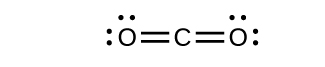
(b) 1 σ 2 π;
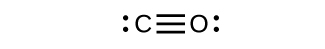
Hybrid Atomic Orbitals
Chapter Exercises
Sulfuric acid is manufactured by a series of reactions represented by the following equations:
\(\ce{S8}(s)+\ce{8O2}(g)⟶\ce{8SO2}(g)\)
\(\ce{2SO2}(g)+\ce{O2}(g)⟶\ce{2SO3}(g)\)
\(\ce{SO3}(g)+\ce{H2O}(l)⟶\ce{H2SO4}(l)\)
Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following:
(a) circular S8 molecule
(b) SO2 molecule
(c) SO3 molecule
(d) H2SO4 molecule (the hydrogen atoms are bonded to oxygen atoms)
(a) Each S has a bent (109°) geometry, sp3

(b) Bent (120°), sp2

(c) Trigonal planar, sp2

(d) Tetrahedral, sp3

For many years after they were discovered, it was believed that the noble gases could not form compounds. Now we know that belief to be incorrect. A mixture of xenon and fluorine gases, confined in a quartz bulb and placed on a windowsill, is found to slowly produce a white solid. Analysis of the compound indicates that it contains 77.55% Xe and 22.45% F by mass.
(a) What is the formula of the compound?
(b) Write a Lewis structure for the compound.
(c) Predict the shape of the molecules of the compound.
(d) What hybridization is consistent with the shape you predicted?
(a) XeF2
(b)

(c) linear
(d) sp3d
Write Lewis structures for NF3 and PF5. On the basis of hybrid orbitals, explain the fact that NF3, PF3, and PF5 are stable molecules, but NF5 does not exist.

Multiple Bonds
Chapter Exercises
A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers.
(a) Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
(b) Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
(c) Describe the atomic orbitals that form the π bonds in the molecule.
(a)

(b) The terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized. (c) Each of the two π bonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital.
Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
(a) ClNO (N is the central atom)
(b) CS2
(c) Cl2CO (C is the central atom)
(a) sp2; (b) sp; (c) sp2
Draw the orbital diagram for carbon in CO2 showing how many carbon atom electrons are in each orbital.

Each of the four electrons is in a separate orbital and overlaps with an electron on an oxygen atom.
Molecular Orbital Theory
Chapter Exercises
Determine the bond order of each member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond.
(a) H2, \(\ce{H2+}\), \(\ce{H2-}\)
(b) O2, \(\ce{O2^2+}\), \(\ce{O2^2-}\)
(a) H2 bond order = 1, \(\ce{H2+}\) bond order = 0.5, \(\ce{H2-}\) bond order = 0.5, strongest bond is H2; (b) O2 bond order = 2, \(\ce{O2^2+}\) bond order = 3; \(\ce{O2^2-}\) bond order = 1, strongest bond is \(\ce{O2^2+}\)


