Introduction
It's possible that the fuel for the car you drive thirty years from now will come from the back end of a panda. Not literally, of course – but it just might turn out that future biofuel technology will be derived in part from the stuff that workers have to clean out of the enclosure housing Ya Ya and Le Le, the two resident pandas at the Memphis Zoo in . At least, that's the hope of Dr. Ashli Brown, a biochemistry professor at Tennessee State University.
First, a little background. If you are like most people in the United States, you are already burning ethanol every time you drive: in 2012, the U.S. Department of Energy reports that over 13 million gallons of ethanol were sold at gas stations nationwide, most often as a 10% mixture along with 90% conventional gasoline. The ethanol we burn today is made by fermenting the sugars present in edible corn. The use of corn ethanol, while a significant step forward in the effort to move away from petroleum fuels and towards carbon-neutral, renewable energy sources, is far from a permanent, sustainable solution to the world's ever-increasing energy needs. Growing corn crops requires a lot of energy and expense, from running the large equipment used to plow and harvest the fields, to manufacturing and applying pesticides and fertilizers, all the way to trucking the corn to the ethanol plant. In fact, some calculation methods suggest that more energy goes into producing a gallon of corn-based ethanol than is released when the ethanol is burned.
Moreover, growing corn requires a lot of water, and takes up land which otherwise could be used for growing food, or preserved as a natural habitat. A recent study by scientists in South Dakota reported that between 2006 and 2011, a full 1.3 million acres of wetland and prairie were plowed over and converted to biofuel crop production in five midwestern states.
What would be much better in the long run is if we could produce ethanol or other biofuels not from resource-intensive food crops like corn, but from non-edible plant materials: grasses, trees, and agricultural byproducts such as the cobs and stalks from corn plants. Switchgrass, for example, is a native North American prairie grass that is thought to have high potential for biofuel production.
So if we can make ethanol from corn, couldn't we just change over to switchgrass using the same technology?
Unfortunately, it's not nearly that simple. Ethanol is made by 'feeding' glucose to living yeast cells, allowing them to break down the sugar into ethanol – a metabolic process called fermentation. Corn kernels contain sugar in the form of starch, a polysaccharide of linked glucose molecules. Enzymes called 'amylases' are used to break up the starch polymer into individual glucose molecules (as well as two-glucose units called cellobiose), which are then fermented by the yeast.
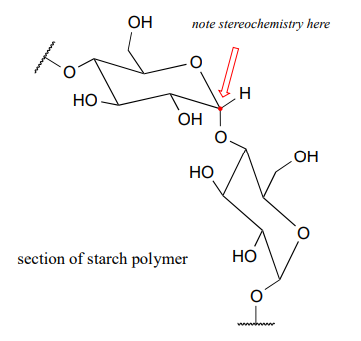
The rest of the corn plant – the stalks, leaves, and cobs – is composed in large part of another glucose polymer called cellulose.
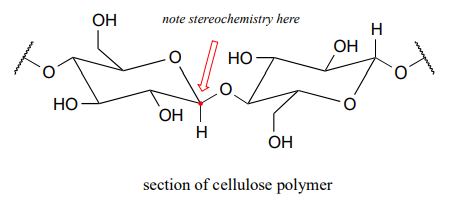
Cellulose is a major component of plant cell walls, and is the most abundant organic compound on the planet - an enormous source of glucose for fermentation! The problem, from a renewable energy perspective, is how to get at the glucose monomers that make up the polymer. Look closely at the bond connecting two glucose monomers in starch, and then compare it to the same bond in cellulose. They both link the same two carbons of glucose, but with opposite stereochemistry. Recall that enzymes are very sensitive to the stereochemical configuration of their substrate molecules. It should come as no surprise, then, that the amylase enzymes which are so efficient at breaking apart starch are completely ineffective at breaking apart cellulose. Other enzymes, known as cellulases, are needed for this job. These enzymes do exist in nature: just think about what happens to tree branches, leaves, and other cellulose-rich plant matter that lies on the forest floor. These slowly rot away, the cellulose broken apart by cellulase enzymes in microscopic fungi.
The key word here, though, is 'slowly'. Fungi living on the forest floor are not in any great hurry to degrade the leaves and wood around them – the cellulose is not going anywhere. Fungal cellulases are, comparatively speaking, very slow, inefficient enzymes. Herein lies the biggest challenge to the development of economically viable production of ethanol from cellulosic sources such as switchgrass or wood. Breaking the glucose-glucose bonds in cellulose is the main bottleneck in the whole process.
This is where the pandas come in.
Pandas live primarily on a diet of bamboo, obtaining their energy from the cellulose in the plant. Like other plant-eaters such as cows, horses, and sheep, pandas do not make their own cellulase enzymes. Rather, they rely on a diverse population of symbiotic microbes inhabiting their digestive tracts to do the job of cellulose digestion for them. Unlike the microbes living the slow-paced lifestyle of the forest floor, though, the panda's microbes don't have a lot of time to spare - the food is moving through the system pretty quickly. In theory, evolutionary pressure should have resulted in panda-gut microbes with speedy cellulase enzymes, and that is what Dr. Ashli Brown at Tennessee State was hoping to find as she and her research students analyzed panda feces from the Memphis Zoo. They have had some success: at the fall, 2013 meeting of the American Chemical Society, Dr. Brown announced that her group, working in cooperation with colleagues at the University of Wisconsin, had found over forty cellulose-digesting bacteria, courtesy of Ya Ya and Le Le. The next step is to clone the cellulase- encoding genes, use the DNA to produce recombinant enzyme, and see just how fast they are.
Other less cuddly and photogenic animals are also being studied with similar goals in mind. Dr. Falk Warnecke, working at the U.S. Department of Energy Joint Genome Institute in Northern California, has been investigating the microbes that live in the guts of wood-eating termites, and many other researchers around the world are interested in the symbiotic bugs which inhabit the rumen of cows and sheep.
The problematic chemical reaction catalyzed by cellulase enzymes is, in organic chemistry terminology, an 'acetal hydrolysis'. Acetals are derived from aldehydes. The reactions that occur at the carbonyl carbon of aldehydes and ketones is absolutely central to the chemistry of carbohydrates such as starch and cellulose, and it is this chemistry that is the subject of the chapter we are about to begin.





