Resonance effects involving aromatic structures can have a dramatic influence on acidity and basicity. Notice, for example, the difference in acidity between phenol and cyclohexanol.
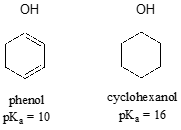
Looking at the conjugate base of phenol, we see that the negative charge can be delocalized by resonance to three different carbons on the aromatic ring.
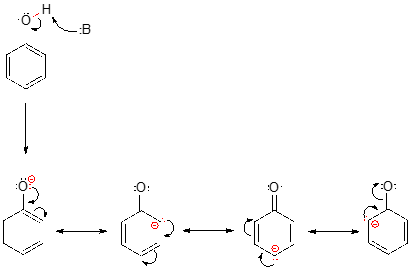
Although these are all minor resonance contributors (negative charge is placed on a carbon rather than the more electronegative oxygen), they nonetheless have a significant effect on the acidity of the phenolic proton. Essentially, the benzene ring is acting as an electron-withdrawing group by resonance.
As we begin to study in detail the mechanisms of biological organic reactions, we’ll see that the phenol side chain of the amino acid tyrosine (see table 5 at the back of the book), with its relatively acidic \(pK_a\) of 9-10, often acts as a catalytic proton donor/acceptor in enzyme active sites.
Draw the conjugate base of 2-napthol (the major resonance contributor), and on your drawing indicate with arrows all of the atoms to which the negative charge can be delocalized by resonance.

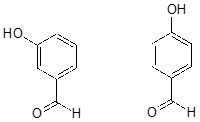
The base-stabilizing effect of an aromatic ring can be accentuated by the presence of an additional electron-withdrawing substituent, such as a carbonyl. For the conjugate base of the phenol derivative below, an additional resonance contributor can be drawn in which the negative formal charge is placed on the carbonyl oxygen.
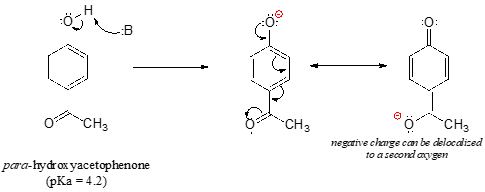
Now the negative charge on the conjugate base can be spread out over two oxygens (in addition to three aromatic carbons). The phenol acid therefore has a \(pK_a\) similar to that of a carboxylic acid, where the negative charge on the conjugate base is also delocalized to two oxygen atoms. The ketone group is acting as an electron withdrawing group - it is 'pulling' electron density towards itself, through both inductive and resonance effects.
The position of the electron-withdrawing substituent relative to the phenol hydroxyl is very important in terms of its effect on acidity. Which of the two substituted phenols below is more acidic? Use resonance drawings to explain your answer.
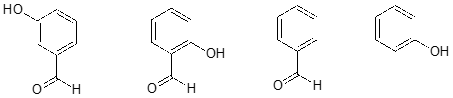
Rank the four compounds below from most acidic to least.
Nitro groups are very powerful electron-withdrawing groups. The phenol derivative picric acid has a pKa of 0.25, lower than that of trifluoroacetic acid.
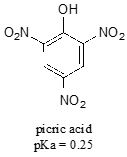
Use a resonance argument to explain why picric acid has such a low pKa.
Consider the acidity of 4-methoxyphenol, compared to phenol.
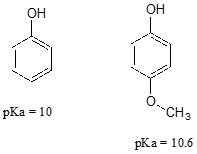
Notice that the methoxy group increases the pKa of the phenol group - it makes it less acidic. Why is this? At first inspection, you might assume that the methoxy substituent, with its electronegative oxygen, would be an electron-withdrawing group by induction. That is correct, but only to a point. The oxygen atom does indeed exert an electron-withdrawing inductive effect, but the lone pairs on the oxygen cause the exact opposite effect – the methoxy group is an electron-donating group by resonance. A resonance contributor can be drawn in which a formal negative charge is placed on the carbon adjacent to the negatively-charged phenolate oxygen.
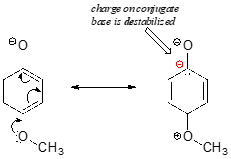
Because of like-charge repulsion, this destabilizes the negative charge on the phenolate oxygen, making it more basic. It may help to visualize the methoxy group ‘pushing’ electrons towards the lone pair electrons of the phenolate oxygen, causing them to be less 'comfortable' and more reactive.
When resonance and induction compete, resonance usually wins!
The example above is a somewhat confusing but quite common situation in organic chemistry - a functional group, in this case a methoxy group, is exerting both an inductive effect and a resonance effect, but in opposite directions (the inductive effect is electron-withdrawing, the resonance effect is electron-donating). As stated earlier, as a general rule a resonance effect is more powerful than an inductive effect - so overall, the methoxy group is acting as an electron donating group.
Rank the three compounds below from lowest pKa to highest, and explain your reasoning. Hint - think about both resonance and inductive effects!