1.2: Drawing organic structures
- Last updated
- Save as PDF
- Page ID
- 170399
Formal Charges
Now that you have had a chance to go back to your introductory chemistry textbook to review some basic information about atoms, orbitals, bonds, and molecules, let's direct our attention a little more closely to the idea of charged species. You know that an ion is a molecule or atom that has an associated positive or negative charge. Copper, for example, can be found in both its neutral state (Cu0, which is the metal), or in its Cu+2 state, as a component of an ionic compound like copper carbonate (CuCO3), the green substance called 'patina' that forms on the surface of copper objects.
Organic molecules can also have positive or negative charges associated with them. Consider the Lewis structure of methanol, CH3OH (methanol is the so-called ‘wood alcohol’ that unscrupulous bootleggers sometimes sold during the prohibition days in the 1920's, often causing the people who drank it to go blind). Methanol itself is a neutral molecule, but can lose a proton to become a molecular anion (CH3O-), or gain a proton to become a molecular cation (CH3OH2+).
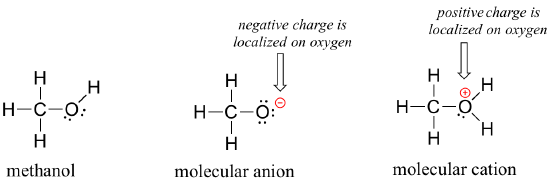
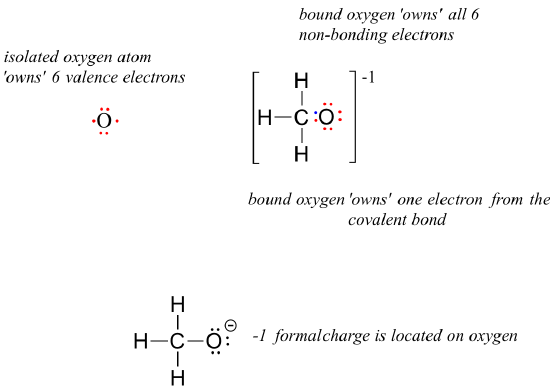
The molecular anion and cation have overall charges of -1 and +1, respectively. But we can be more specific than that - we can also state for each molecular ion that a formal charge is located specifically on the oxygen atom, rather than on the carbon or any of the hydrogen atoms.
Figuring out the formal charge on different atoms of a molecule is a straightforward process - it’s simply a matter of adding up valence electrons.
A unbound oxygen atom has 6 valence electrons. When it is bound as part of a methanol molecule, however, an oxygen atom is surrounded by 8 valence electrons: 4 nonbonding electrons (two 'lone pairs') and 2 electrons in each of its two covalent bonds (one to carbon, one to hydrogen). In the formal charge convention, we say that the oxygen 'owns' all 4 nonbonding electrons. However, it only 'owns' one electron from each of the two covalent bonds, because covalent bonds involve the sharing of electrons between atoms. Therefore, the oxygen atom in methanol owns 2 + 2 + (½ x 4) = 6 valence electrons.
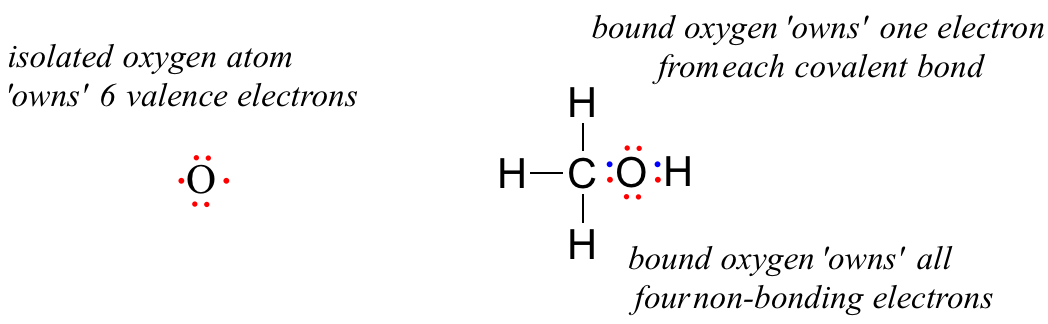
The formal charge on an atom is calculated as the number of valence electrons owned by the isolated atom minus the number of valence electrons owned by the bound atom in the molecule:
Determining formal charge on an atom
formal charge =
(number of valence electrons owned by the isolated atom)
- (number of valence electrons owned by the bound atom)
or . . .
formal charge =
(number of valence electrons owned by the isolated atom)
- (number of non-bonding electrons on the bound atom)
- ( ½ the number of bonding electrons on the bound atom)
Using this formula for the oxygen atom of methanol, we have:
formal charge on oxygen =
(6 valence electrons on isolated atom)
- (4 non-bonding electrons)
- (½ x 4 bonding electrons)
= 6 - 4 - 2 = 0. Thus, oxygen in methanol has a formal charge of zero (in other words, it has no formal charge).
How about the carbon atom in methanol? An isolated carbon owns 4 valence electrons. The bound carbon in methanol owns (½ x 8) = 4 valence electrons:
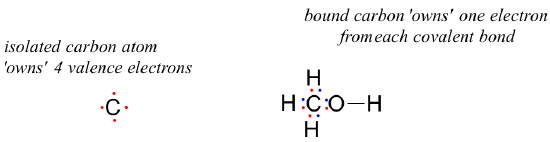
formal charge on carbon =
(4 valence electron on isolated atom)
- (0 nonbonding electrons)
- (½ x 8 bonding electrons)
= 4 - 0 - 4 = 0. So the formal charge on carbon is zero.
For each of the hydrogens in methanol, we also get a formal charge of zero:
formal charge on hydrogen =
(1 valence electron on isolated atom)
- (0 nonbonding electrons)
- (½ x 2 bonding electrons)
= 1 - 0 - 1 = 0
Now, let's look at the cationic form of methanol, CH3OH2+. The bonding picture has not changed for carbon or for any of the hydrogen atoms, so we will focus on the oxygen atom.
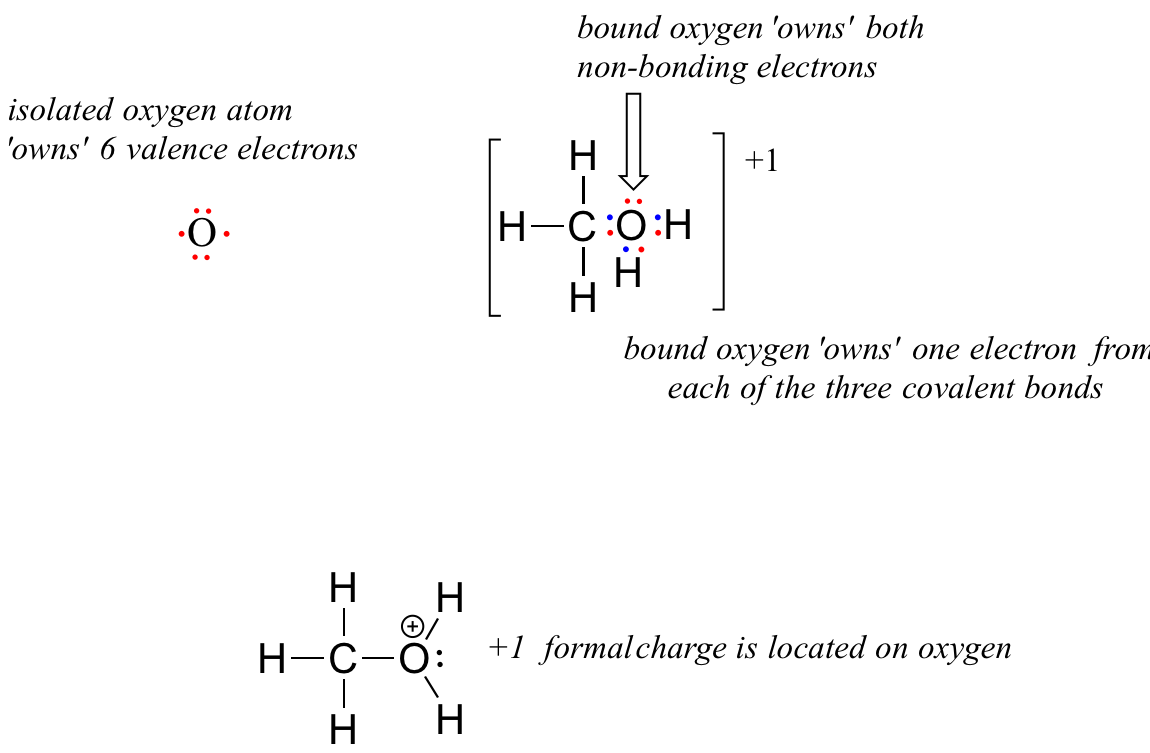
The oxygen owns 2 non-bonding electrons and 3 bonding elections, so the formal charge calculations becomes:
formal charge on oxygen =
(6 valence electrons in isolated atom)
- (2 non-bonding electrons)
- (½ x 6 bonding electrons)
= 6 - 2 - 3 = 1. A formal charge of +1 is located on the oxygen atom.
For methoxide, the anionic form of methanol, the calculation for the oxygen atom is:
formal charge on oxygen =
(6 valence electrons in isolated atom)
- (6 non-bonding electrons)
- (½ x 2 bonding electrons)
= 6 - 6 - 1 = -1. A formal charge of -1 is located on the oxygen atom.
A very important rule to keep in mind is that the sum of the formal charges on all atoms of a molecule must equal the net charge on the whole molecule.
When drawing the structures of organic molecules, it is very important to show all non-zero formal charges, being clear about where the charges are located. A structure that is missing non-zero formal charges is not correctly drawn, and will probably be marked as such on an exam!
At this point, thinking back to what you learned in general chemistry, you are probably asking “What about dipoles? Doesn’t an oxygen atom in an O-H bond ‘own’ more of the electron density than the hydrogen, because of its greater electronegativity?” This is absolutely correct, and we will be reviewing the concept of bond dipoles later on. For the purpose of calculating formal charges, however, bond dipoles don’t matter - we always consider the two electrons in a bond to be shared equally, even if that is not an accurate reflection of chemical reality. Formal charges are just that - a formality, a method of electron book-keeping that is tied into the Lewis system for drawing the structures of organic compounds and ions. Later, we will see how the concept of formal charge can help us to visualize how organic molecules react.
Finally, don't be lured into thinking that just because the net charge on a structure is zero there are no atoms with formal charges: one atom could have a positive formal charge and another a negative formal charge, and the net charge would still be zero. Zwitterions, such as amino acids, have both positive and negative formal charges on different atoms:
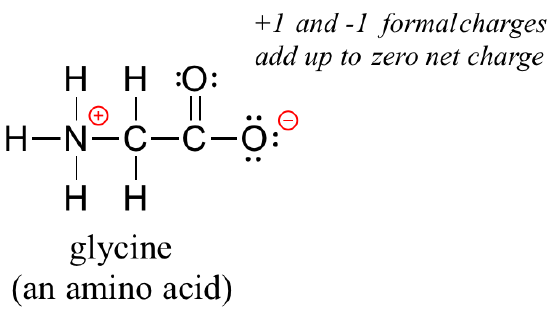
Even though the net charge on glycine is zero, it is still neccessary to show the location of the positive and negative formal charges.
Exercise 1.4
Fill in all missing lone pair electrons and formal charges in the structures below. Assume that all atoms have a complete valence shell of electrons. Net charges are shown outside the brackets.
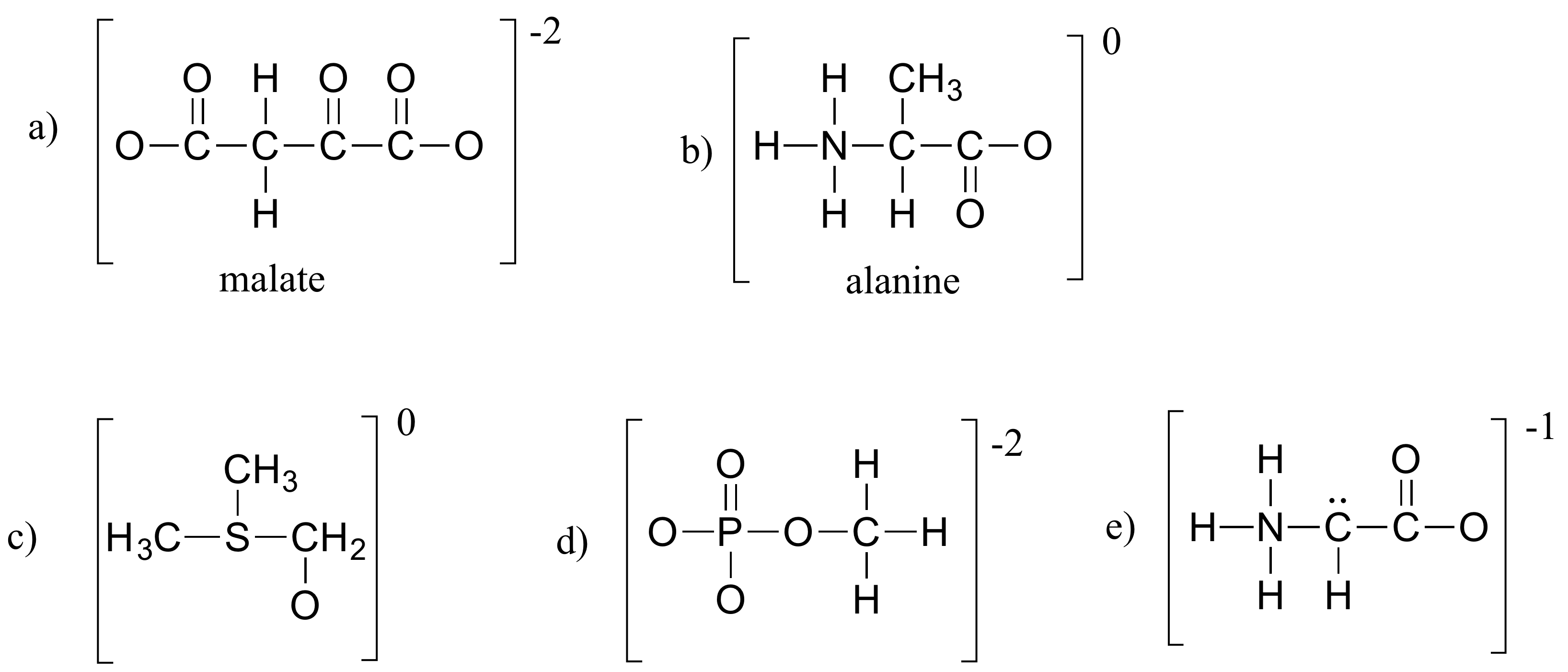
Solutions to exercises
Common bonding patterns in organic structures
The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. But as you can imagine, these methods become unreasonably tedious and time-consuming when you start dealing with larger structures. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons.
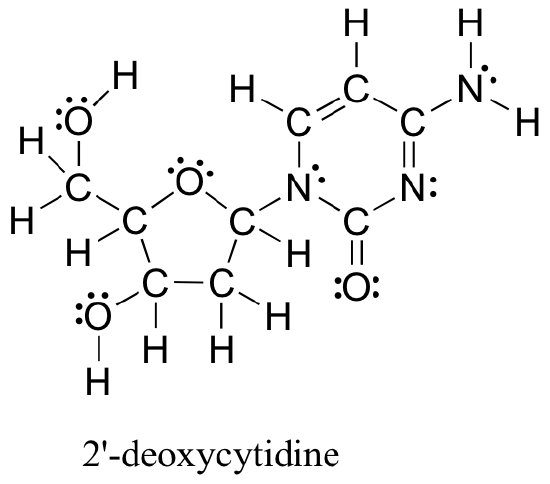
And yet, as organic chemists, and especially as organic chemists dealing with biological molecules, you will be expected soon to draw the structure of large molecules such as this on a regular basis. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns.
Let’s start with carbon, the most important element for organic chemists. Carbon is said to be tetravalent, meaning that it tends to form four bonds. If you look at the simple structures of methane, methanol, ethane, ethene, and ethyne in the figures from the previous section, you should quickly recognize that in each molecule, the carbon atom has four bonds, and a formal charge of zero.
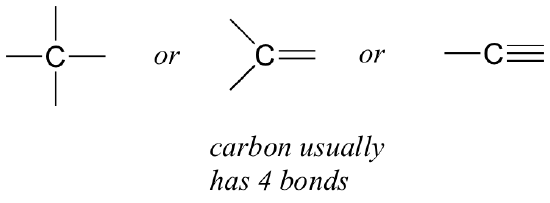
This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions.
In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. If a carbon has only three bonds and an unfilled valence shell (in other words, if it does not fulfill the octet rule), it will have a positive formal charge.
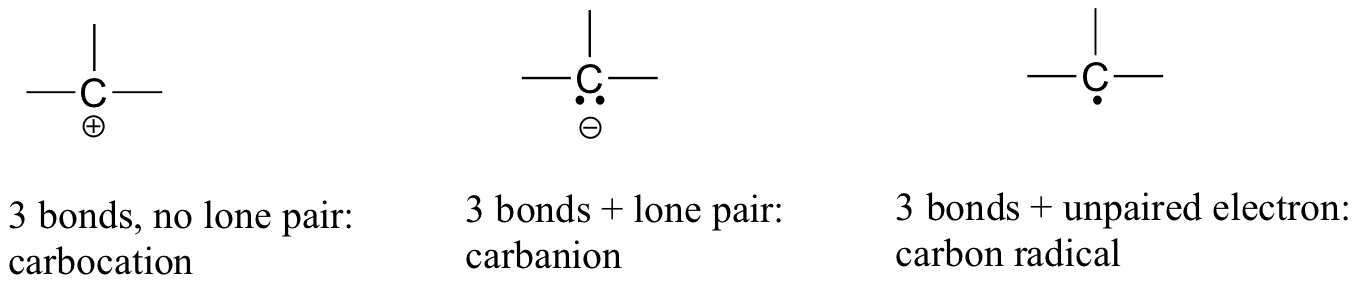
If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. Another possibility is a carbon with three bonds and a single, unpaired (free radical) electron: in this case, the carbon has a formal charge of zero. (One last possibility is a highly reactive species called a ‘carbene’, in which a carbon has two bonds and one lone pair of electrons, giving it a formal charge of zero. You may encounter carbenes in more advanced chemistry courses, but they will not be discussed any further in this book).
You should certainly use the methods you have learned to check that these formal charges are correct for the examples given above. More importantly, you will need, before you progress much further in your study of organic chemistry, to simply recognize these patterns (and the patterns described below for other atoms) and be able to identify by quick inspection carbons that bear positive and negative formal charges.
The pattern for hydrogens is easy: hydrogen atoms have only one bond, and no formal charge. The exceptions to this rule are the proton, H+, and the hydride ion, H-, which is a proton plus two electrons. Because we are concentrating in this book on organic chemistry as applied to living things, however, we will not be seeing ‘naked’ protons and hydrides as such, because they are too reactive to be present in that form in aqueous solution. Nonetheless, the idea of a proton will be very important when we discuss acid-base chemistry, and the idea of a hydride ion will become very important much later in the book when we discuss organic oxidation and reduction reactions. As a rule, though, all hydrogen atoms in organic molecules have one bond, and no formal charge.
Let us next turn to oxygen atoms. Typically, you will see an oxygen bonding in three ways, all of which fulfill the octet rule.
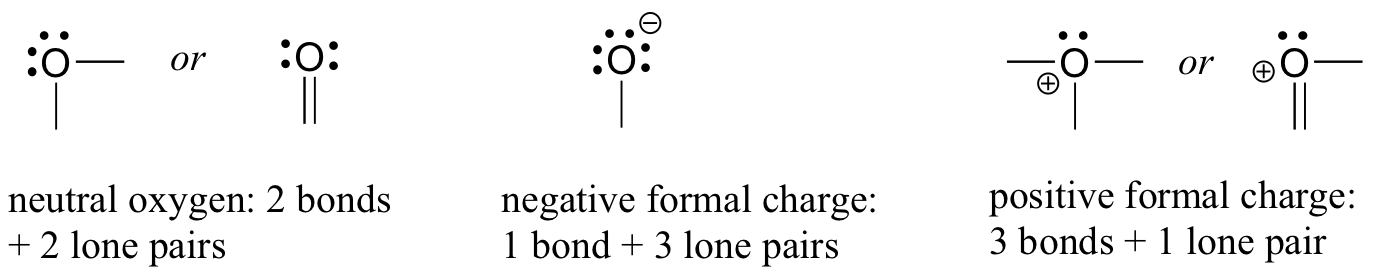
If an oxygen atom t has two bonds and two lone pairs, as in water, it will have a formal charge of zero. If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1.
When we get to our discussion of free radical chemistry in chapter 17, we will see other possibilities, such as where an oxygen atom has one bond, one lone pair, and one unpaired (free radical) electron, giving it a formal charge of zero. For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17.
Nitrogen has two major bonding patterns, both of which fulfill the octet rule:
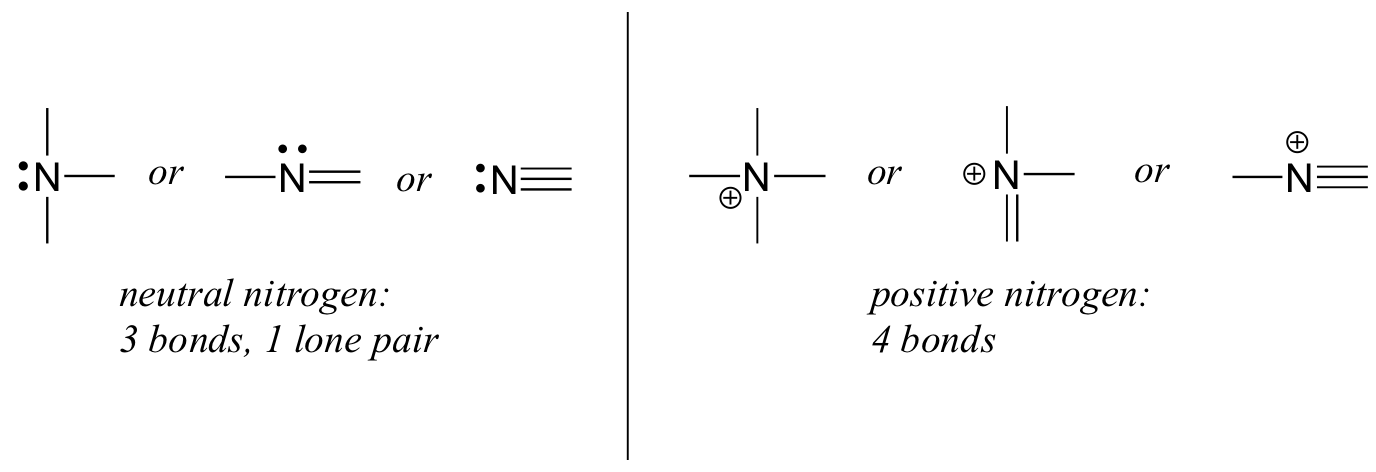
If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If it has four bonds (and no lone pair), it has a formal charge of +1. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs.
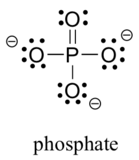
Two third row elements are commonly found in biological organic molecules: sulfur and phosphorus. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero. Remember that atoms of elements in the third row and below in the periodic table have 'expanded valence shells' with d orbitals available for bonding, and the the octet rule does not apply.
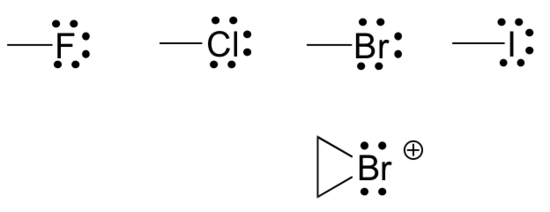
Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. Halogens in organic compounds usually are seen with one bond, three lone pairs, and a formal charge of zero. Sometimes, especially in the case of bromine, we will encounter reactive species in which the halogen has two bonds (usually in a three-membered ring), two lone pairs, and a formal charge of +1.
These rules, if learned and internalized so that you don’t even need to think about them, will allow you to draw large organic structures, complete with formal charges, quite quickly.
Once you have gotten the hang of drawing Lewis structures, it is not always necessary to draw lone pairs on heteroatoms, as you can assume that the proper number of electrons are present around each atom to match the indicated formal charge (or lack thereof). Occasionally, though, lone pairs are drawn if doing so helps to make an explanation more clear.
Exercise 1.5
Draw one structure that corresponds to each of the following molecular formulas, using the common bonding patters covered above. Be sure to include all lone pairs and formal charges where applicable, and assume that all atoms have a full valence shell of electrons. More than one correct answer is possible for each, so you will want to check your answers with your instructor or tutor.
a) C5H10O b) C5H8O c) C6H8NO+ d) C4H3O2-
Solutions to exercises
Using the 'line structure' convention
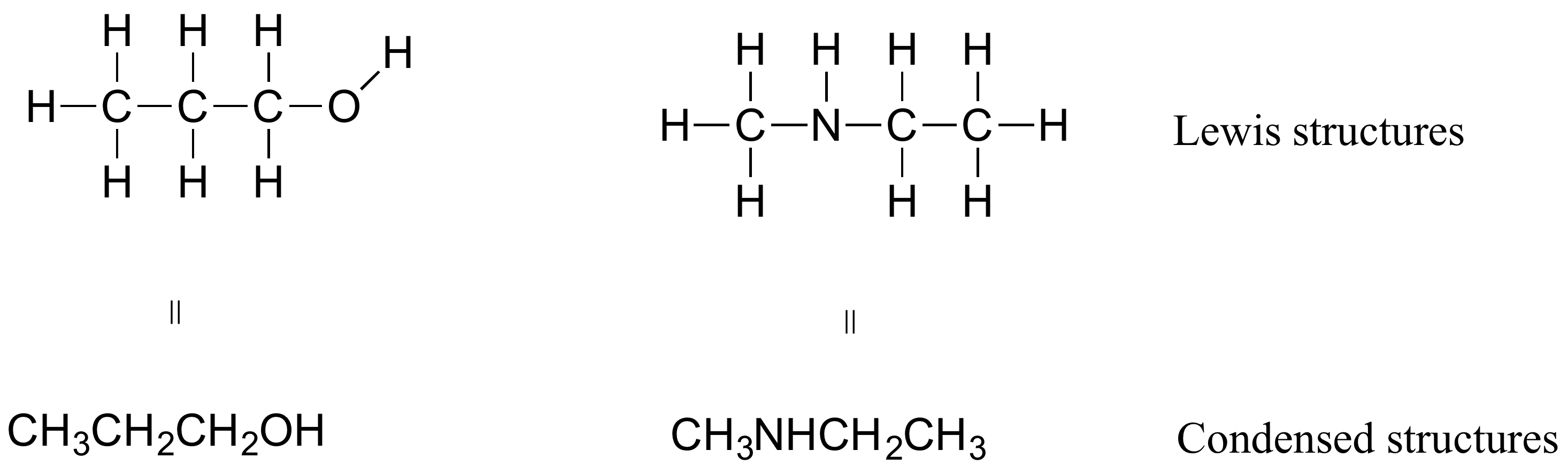
If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. In some sources, you will see condensed structures for smaller molecules instead of full Lewis structures:
More commonly, organic and biological chemists use an abbreviated drawing convention called line structures. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. Carbon atoms are depicted not by a capital C, but by a ‘corner’ between two bonds, or a free end of a bond. Open-chain molecules are usually drawn out in a 'zig-zig' shape. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, they are simply implied (unless a positive formal charge is shown, all carbons are assumed to have a full octet of valence electrons). Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. The following examples illustrate the convention.
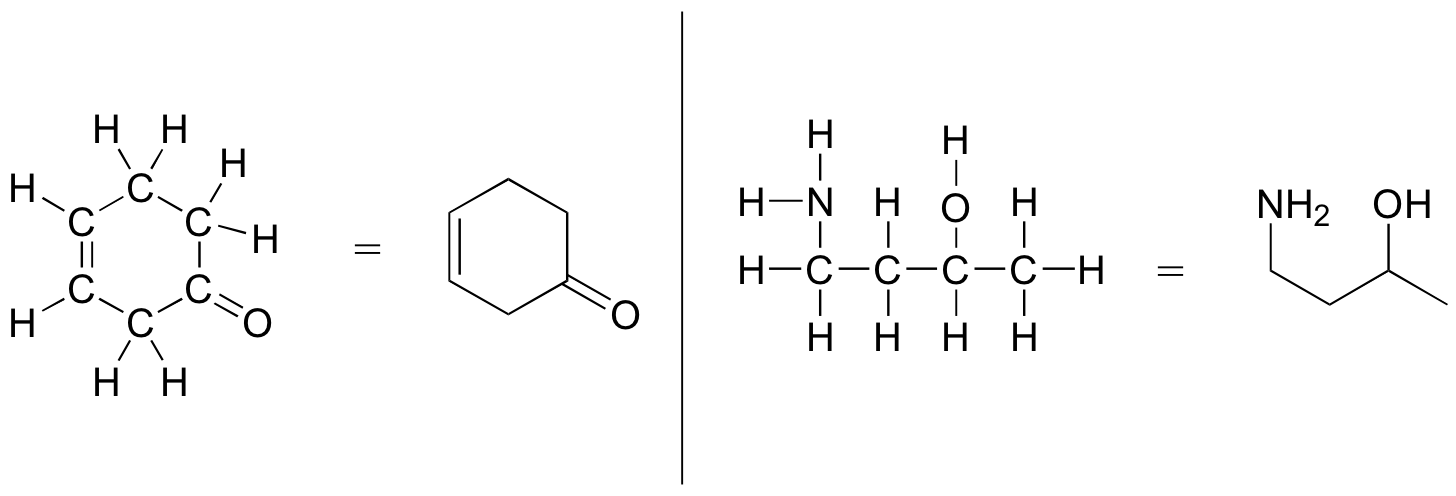
As you can see, the 'pared down' line structure makes it much easier to see the basic structure of the molecule and the locations where there is something other than C-C and C-H single bonds. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures.
Sometimes, one or more carbon atoms in a line structure will be depicted with a capital C, if doing so makes an explanation easier to follow. If you label a carbon with a C, you also must draw in the hydrogens for that carbon.
Exercise 1.6
A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Do this for the structures below.
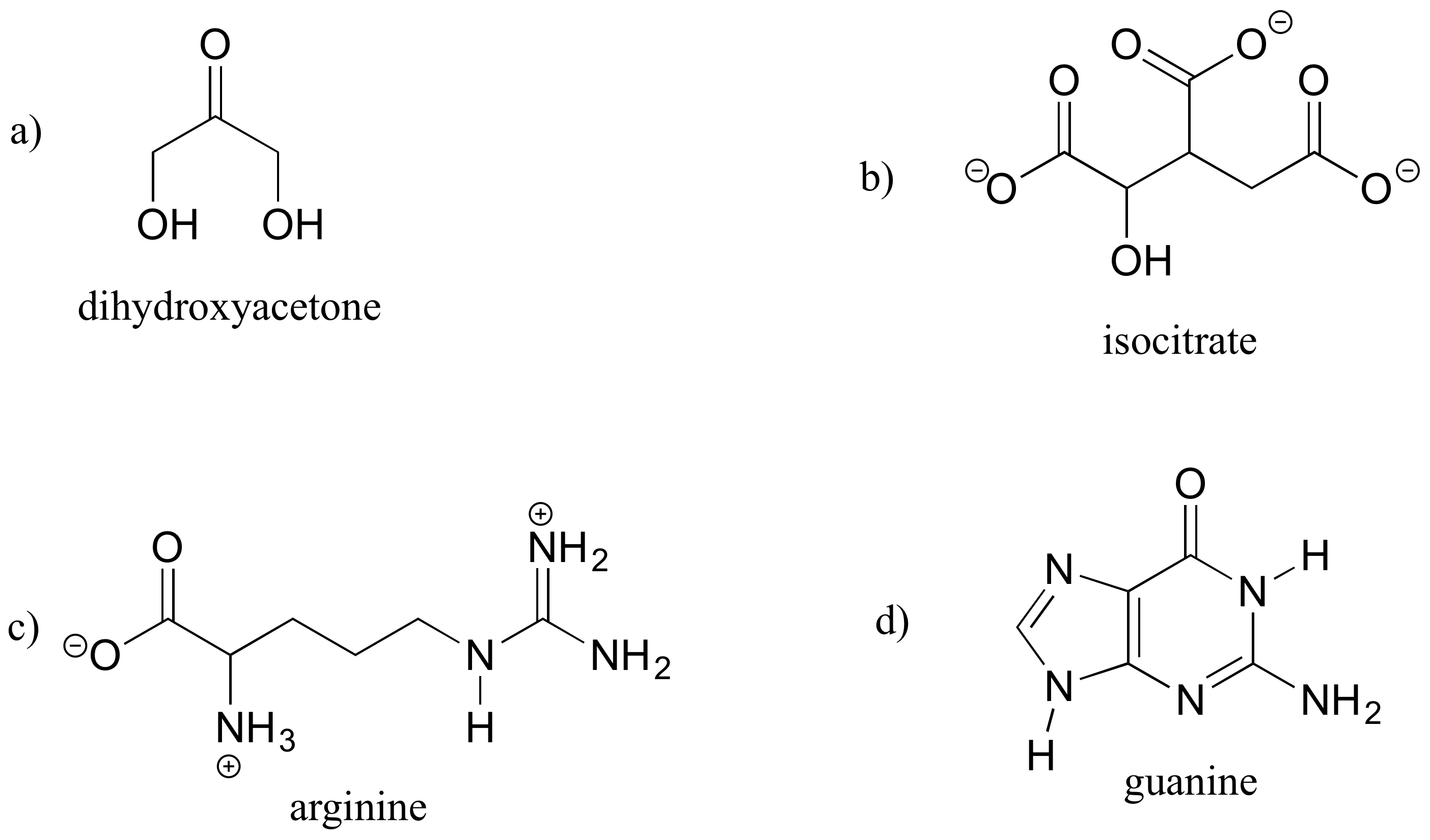
Exercise 1.7
a) Draw a line structure for the DNA base 2-deoxycytidine (the full structure was shown earlier)
b) Draw line structures for histidine (an amino acid) and pyridoxine (Vitamin B6).
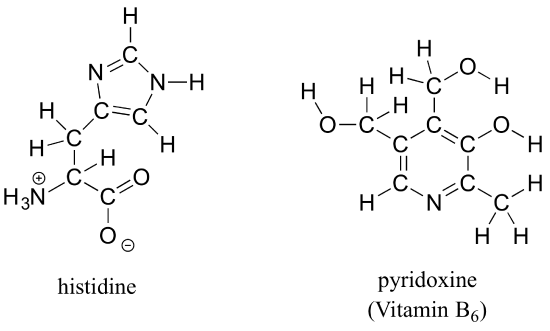
Exercise 1.8
Add non-zero formal charges to the structural drawing below. The net charge is -2.
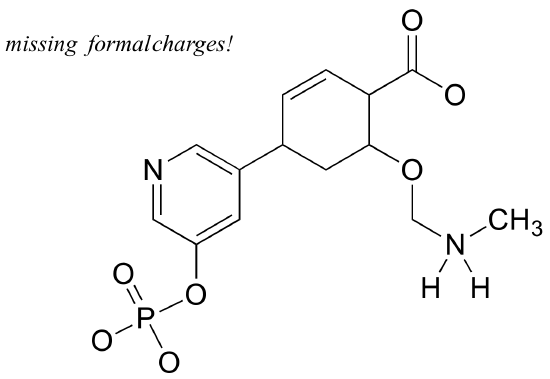
Exercise 1.9
Find, anywhere in chapters 2-17 of this textbook, one example of each of the common bonding patterns specified below. Check your answers with your instructor or tutor.
a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge
b) oxygen with two single bonds, two lone pairs, and zero formal charge
c) oxygen with one double bond, two lone pairs, and zero formal charge
d) nitrogen with one double bond, two single bonds, and a +1 formal charge
e) oxygen with one single bond, three lone pairs, and a negative formal charge
Solutions to exercises
Constitutional isomers
Now that we have reviewed how to draw Lewis structures and learned the line structure shortcut, it is a good time to learn about the concept of constitutional isomers. Imagine if you were asked to draw a structure (Lewis or line) for a compound with the molecular formula C4H10. This would not be difficult - you could simply draw:
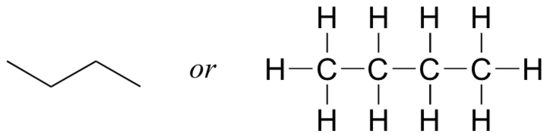
But when you compared your answer with that of a classmate, she may have drawn this structure:
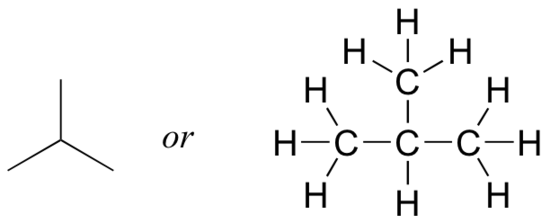
Who is correct? The answer, of course, is that both of you are. A molecular formula only tells you how many atoms of each element are present in the compound, not what the actual atom-to-atom connectivity is. There are often many different possible structures for one molecular formula. Compounds that have the same molecular formula but different connectivity are called constitutional isomers (sometimes the term ‘structural isomer’ is also used). The Greek term ‘iso’ means ‘same’.
Fructose and glucose, two kinds of sugar molecules, are constitutional isomers with the molecular formula C6H12O6.
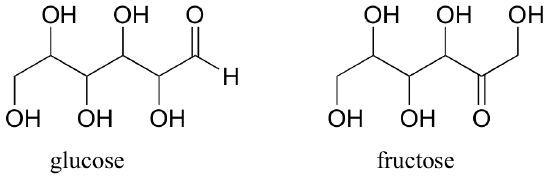
Later, we will see other types of isomers that have the same molecular formula and the same connectivity, but are different in other respects.
Exercise 1.10
Draw a constitutional isomer of ethanol, CH3CH2OH.
Exercise 1.11
Draw all of the possible constitutional isomers with the given molecular formula.
a) C5H12
b) C4H10O
c) C3H9N
Solutions to exercises
Khan Academy video tutorials:
Representing structures of organic molecules
Drawing Lewis Dot structures and determining formal charges
Contributors and Attributions
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


