11.2: Alkanes
- Page ID
- 83131
Skills to Develop
- To identify and name simple (straight-chain) alkanes given formulas and write formulas for straight-chain alkanes given their names.
- To learn how alkane molecules can have branched chains and recognize compounds that are isomers.
We begin our study of organic chemistry with the hydrocarbons, the simplest organic compounds, which are composed of carbon and hydrogen atoms only. As we noted, there are several different kinds of hydrocarbons. They are distinguished by the types of bonding between carbon atoms and the properties that result from that bonding. Hydrocarbons with only carbon-to-carbon single bonds (C–C) and existing as a continuous chain of carbon atoms also bonded to hydrogen atoms are called alkanes (or saturated hydrocarbons). Saturated, in this case, means that each carbon atom is bonded to four other atoms (hydrogen or carbon)—the most possible; there are no double or triple bonds in the molecules.
The word saturated has the same meaning for hydrocarbons as it does for the dietary fats and oils: the molecule has no carbon-to-carbon double bonds (C=C).
We previously introduced the three simplest alkanes—methane (CH4), ethane (C2H6), and propane (C3H8) and they are shown again in Figure \(\PageIndex{1}\).
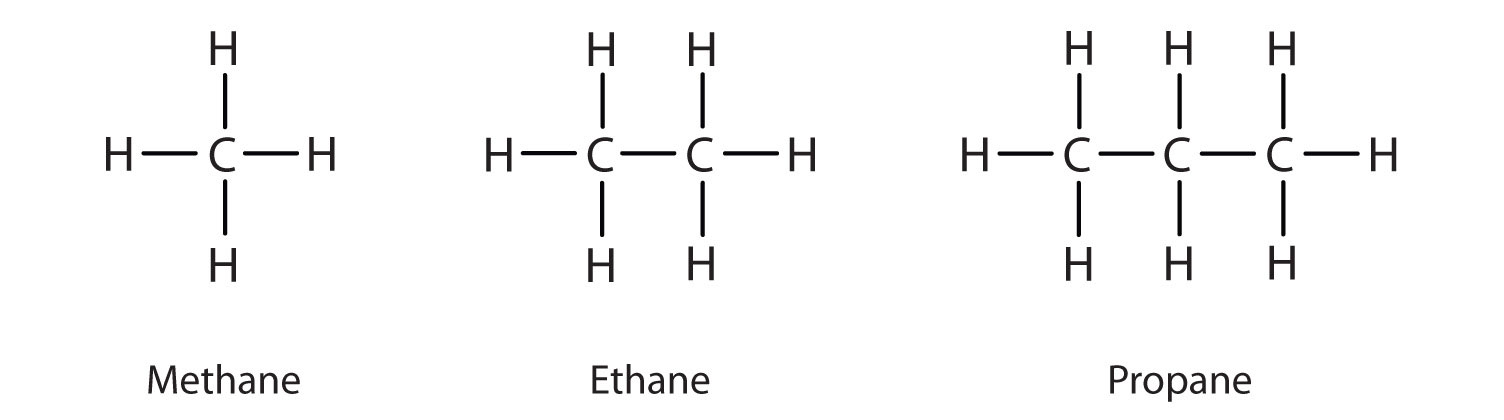
Figure \(\PageIndex{1}\): The Three Simplest Alkanes
The flat representations shown do not accurately portray bond angles or molecular geometry. Methane has a tetrahedral shape that chemists often portray with wedges indicating bonds coming out toward you and dashed lines indicating bonds that go back away from you. An ordinary solid line indicates a bond in the plane of the page. Recall that the VSEPR theory correctly predicts a tetrahedral shape for the methane molecule (Figure \(\PageIndex{2}\)).
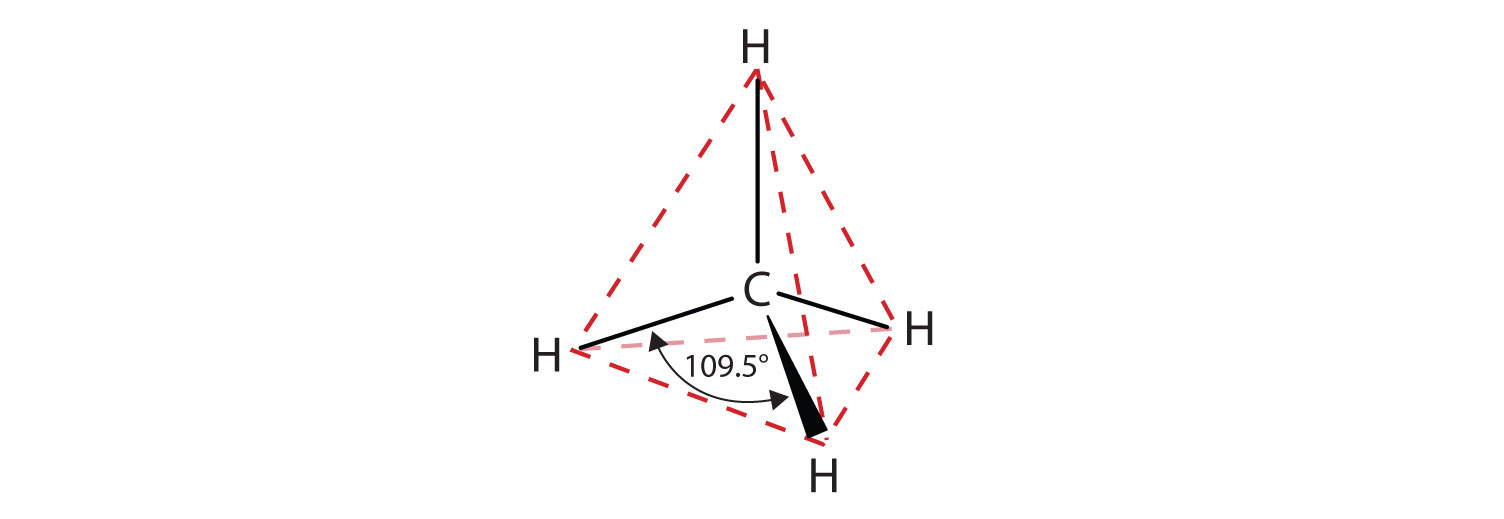
Figure \(\PageIndex{2}\): The Tetrahedral Methane Molecule
Methane (CH4), ethane (C2H6), and propane (C3H8) are the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH2 unit. The first 10 members of this series are given in Table \(\PageIndex{1}\).
| Name | Molecular Formula (CnH2n + 2) | Condensed Structural Formula | Number of Possible Isomers |
|---|---|---|---|
| methane | CH4 | CH4 | — |
| ethane | C2H6 | CH3CH3 | — |
| propane | C3H8 | CH3CH2CH3 | — |
| butane | C4H10 | CH3CH2CH2CH3 | 2 |
| pentane | C5H12 | CH3CH2CH2CH2CH3 | 3 |
| hexane | C6H14 | CH3CH2CH2CH2CH2CH3 | 5 |
| heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 | 9 |
| octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 | 18 |
| nonane | C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | 35 |
| decane | C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | 75 |
Consider the series in Figure \(\PageIndex{3}\). The sequence starts with C3H8, and a CH2 unit is added in each step moving up the series. Any family of compounds in which adjacent members differ from each other by a definite factor (here a CH2 group) is called a homologous series. The members of such a series, called homologs, have properties that vary in a regular and predictable manner. The principle of homology gives organization to organic chemistry in much the same way that the periodic table gives organization to inorganic chemistry. Instead of a bewildering array of individual carbon compounds, we can study a few members of a homologous series and from them deduce some of the properties of other compounds in the series.
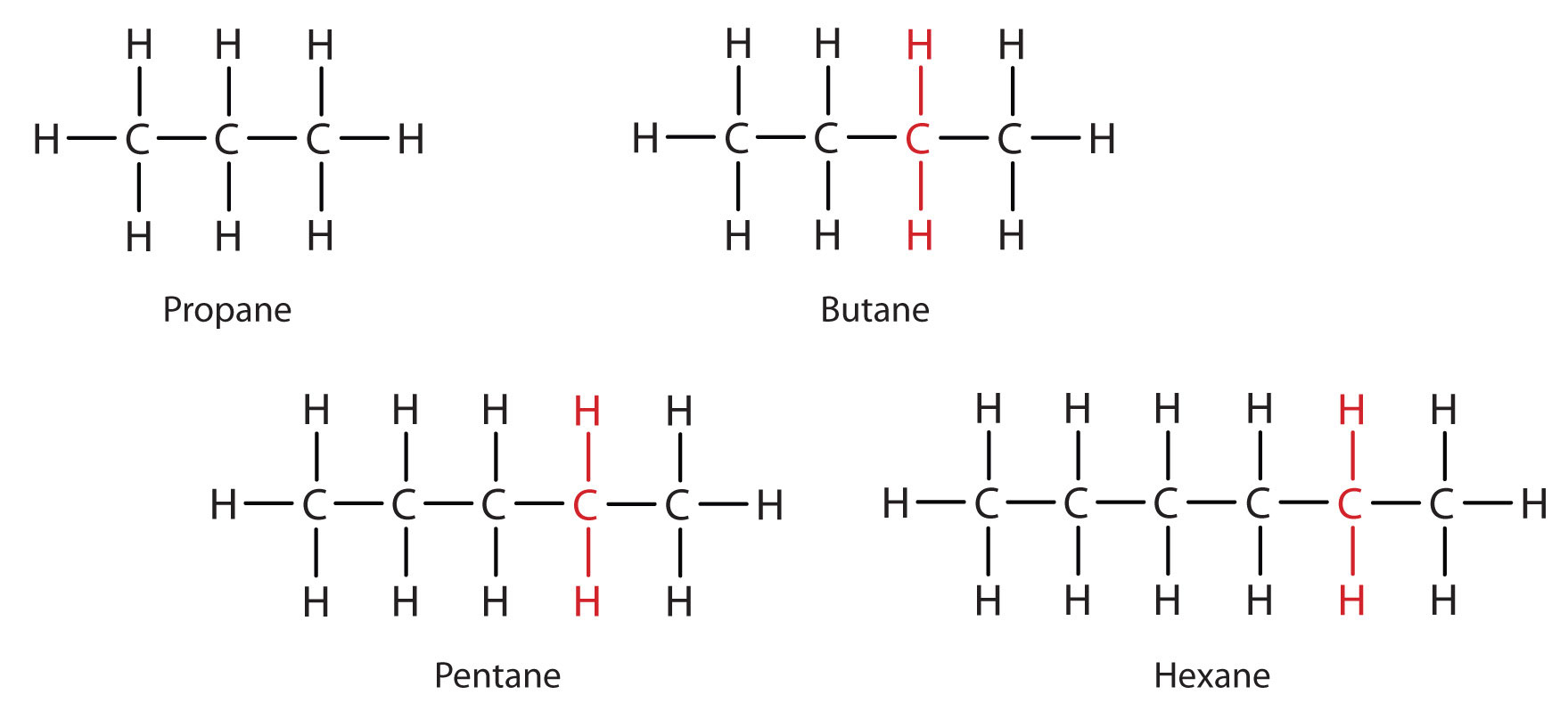
Figure \(\PageIndex{3}\): Members of a Homologous Series. Each succeeding formula incorporates one carbon atom and two hydrogen atoms more than the previous formula.
The principle of homology allows us to write a general formula for alkanes: CnH2n + 2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. For example, an alkane with eight carbon atoms has the molecular formula C8H(2 × 8) + 2 = C8H18.
Example \(\PageIndex{1}\)
Determine the formula for dodecane, an alkane with twelve carbons.
SOLUTION
The general formula will be CnH2n + 2. It has twelve carbons, so n = 12. The number of hydrogens is 2n+2 = 2(12)+2 = 26. The formula is therefore C12H26.Branched-Chain Alkanes
As seen above, we can write the structure of butane (C4H10) by stringing four carbon atoms in a row,
–C–C–C–C–
and then adding enough hydrogen atoms to give each carbon atom four bonds:
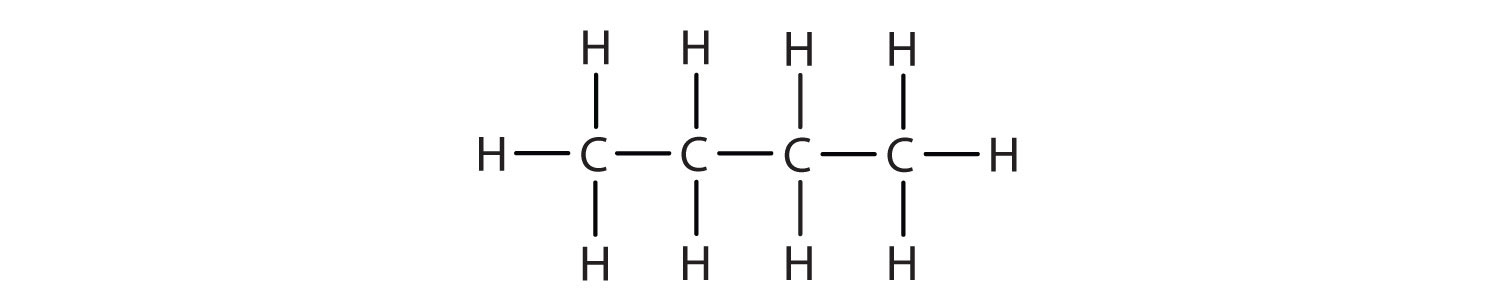
The compound butane has this structure, but there is another way to put 4 carbon atoms and 10 hydrogen atoms together. Place 3 of the carbon atoms in a row and then branch the fourth one off the middle carbon atom:
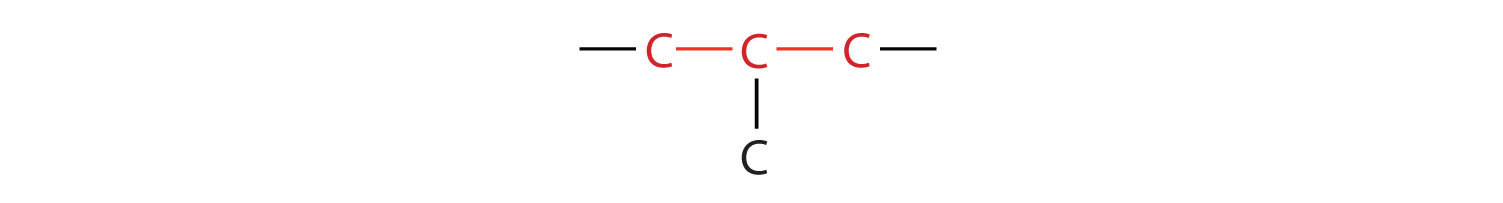
Now we add enough hydrogen atoms to give each carbon four bonds.
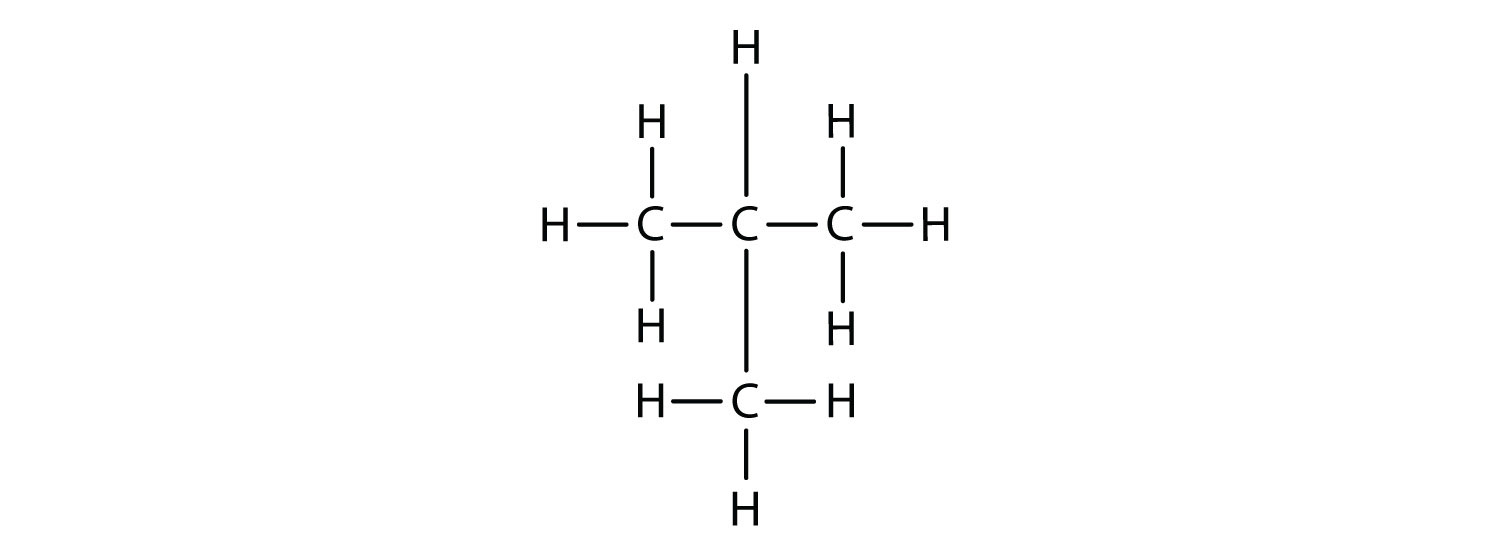
There is a hydrocarbon that corresponds to this structure, which means that two different compounds have the same molecular formula: C4H10. The two compounds have different properties—for example, one boils at −0.5°C; the other at −11.7°C. Different compounds having the same molecular formula are called isomers. The compound with this branched chain is called isobutane (Figure \(\PageIndex{4}\)).
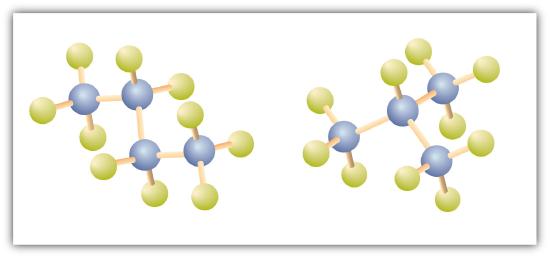
Figure \(\PageIndex{4}\): Butane and Isobutane. The ball-and-stick models of these two compounds show them to be isomers; both have the molecular formula C4H10.
Notice that C4H10 is depicted with a bent chain in Figure \(\PageIndex{1}\). The four-carbon chain may be bent in various ways because the groups can rotate freely about the C–C bonds. However, this rotation does not change the identity of the compound. It is important to realize that bending a chain does not change the identity of the compound; all of the following represent the same compound:
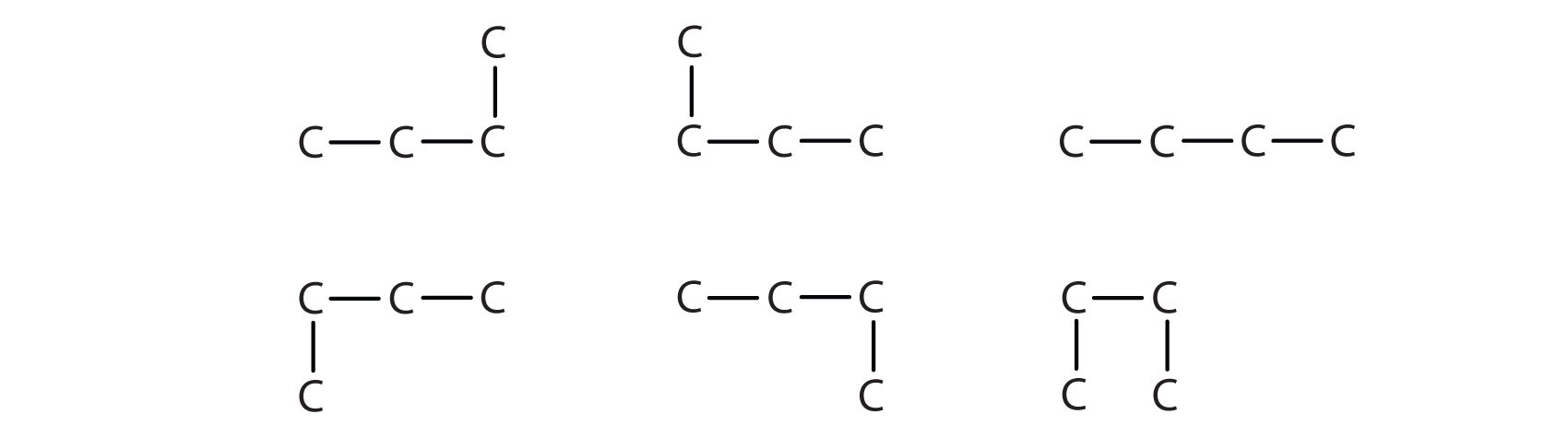
The formula of isobutane shows a continuous chain of three carbon atoms only, with the fourth attached as a branch off the middle carbon atom of the continuous chain.
Unlike C4H10, the compounds methane (CH4), ethane (C2H6), and propane (C3H8) do not exist in isomeric forms because there is only one way to arrange the atoms in each formula so that each carbon atom has four bonds.
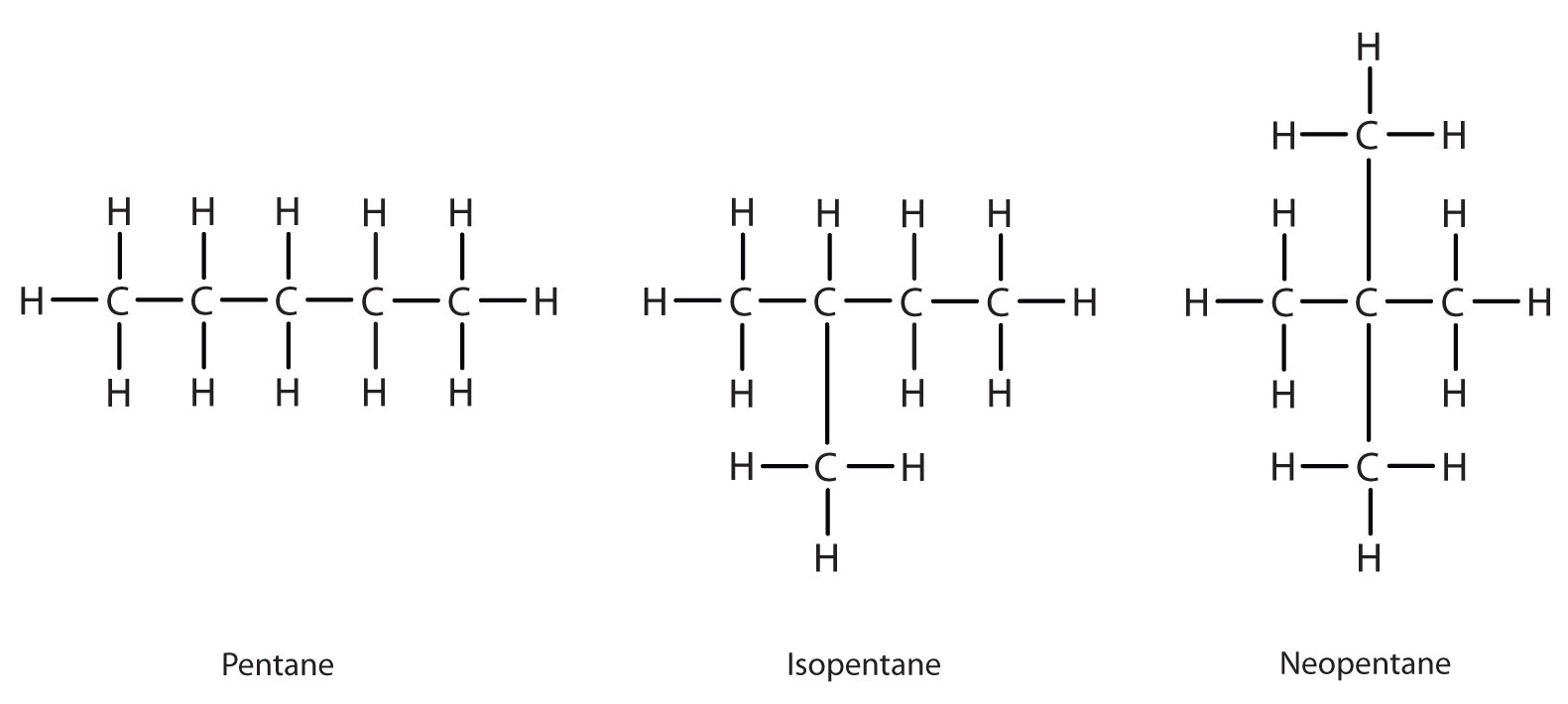
Next beyond C4H10 in the homologous series is pentane. Each compound has the same molecular formula: C5H12. (Table 12.2 has a column identifying the number of possible isomers for the first 10 straight-chain alkanes.) The compound at the far left is pentane because it has all five carbon atoms in a continuous chain. The compound in the middle is isopentane; like isobutane, it has a one CH3 branch off the second carbon atom of the continuous chain. The compound at the far right, discovered after the other two, was named neopentane (from the Greek neos, meaning “new”). Although all three have the same molecular formula, they have different properties, including boiling points: pentane, 36.1°C; isopentane, 27.7°C; and neopentane, 9.5°C.
A continuous (unbranched) chain of carbon atoms is often called a straight chain even though the tetrahedral arrangement about each carbon gives it a zigzag shape. Straight-chain alkanes are sometimes called normal alkanes, and their names are given the prefix n-. For example, butane is called n-butane. We will nt use that prefix here because it is not a part of the system established by the International Union of Pure and Applied Chemistry.
Example \(\PageIndex{2}\)
Indicate whether the structures in each pair represent the same compound, isomers, or neither.
A)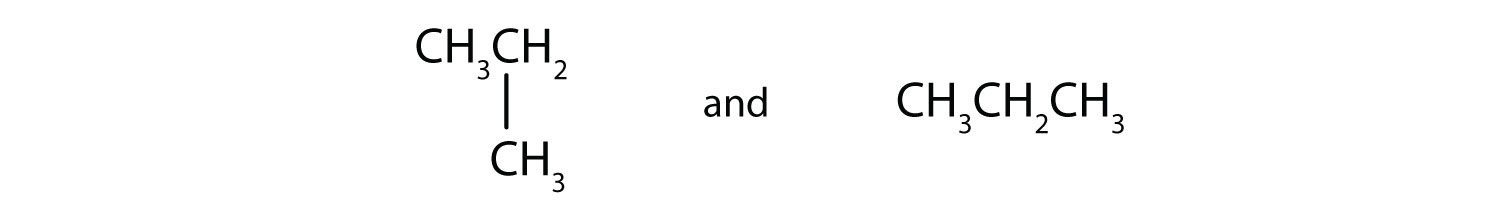
B)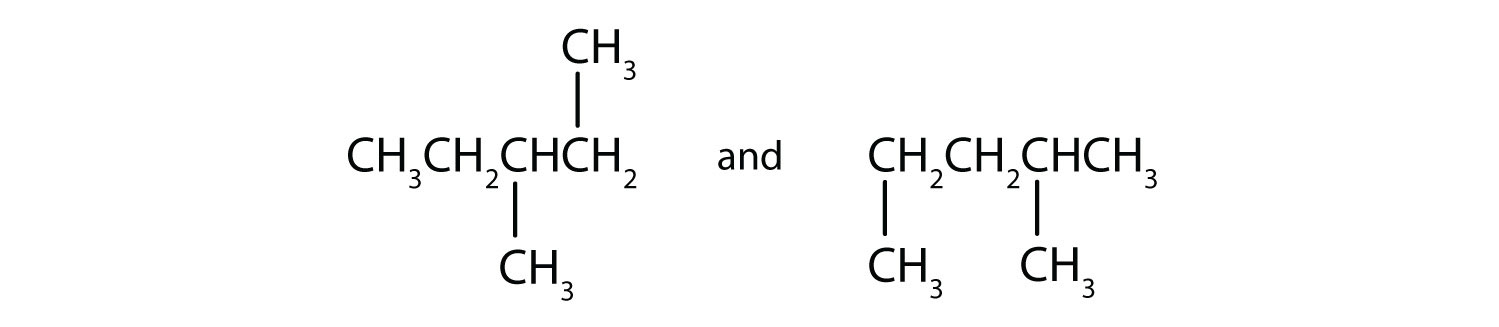
C)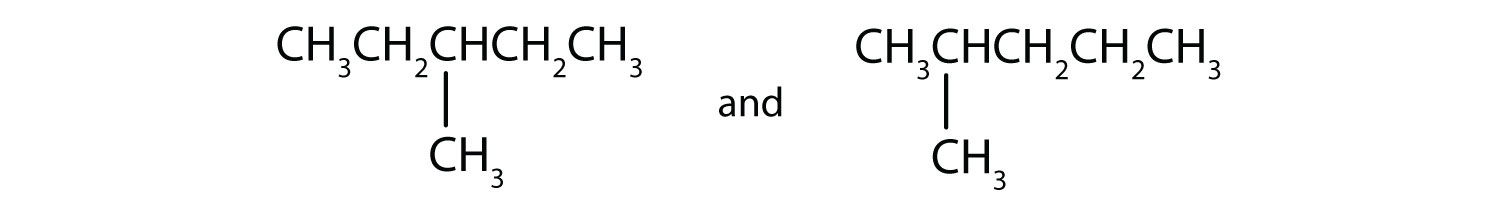
D)
E) 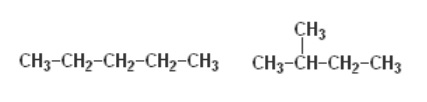
SOLUTION
A) same
B) isomers
C) isomers
D) same
E) isomers
Chemical Properties of Alkanes
Alkane molecules are nonpolar and therefore generally do not react with ionic compounds such as most laboratory acids, bases, oxidizing agents, or reducing agents. Consider butane as an example:
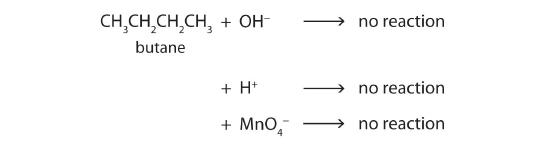
Neither positive ions nor negative ions are attracted to a nonpolar molecule. In fact, the alkanes undergo so few reactions that they are sometimes called paraffins, from the Latin parum affinis, meaning “little affinity.”
One important reactions that the alkanes do undergo is combustion. Nothing happens when alkanes are merely mixed with oxygen (\(O_2\)) at room temperature, but when a flame or spark provides the activation energy, a highly exothermic combustion reaction proceeds vigorously. For methane (CH4), the reaction is as follows:
\[CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O + \text{heat} \label{12.7.1}\]
If the reactants are adequately mixed and there is sufficient oxygen, the only products are carbon dioxide (\(CO_2\)), water (\(H_2O\)), and heat—heat for cooking foods, heating homes, and drying clothes. Because conditions are rarely ideal, however, other products are frequently formed. When the oxygen supply is limited, carbon monoxide (\(CO\)) is a by-product:
\[2CH_4 + 3O_2 \rightarrow 2CO + 4H_2O\label{12.7.2}\]
This reaction is responsible for dozens of deaths each year from unventilated or improperly adjusted gas heaters. (Similar reactions with similar results occur with kerosene heaters.)
Key Takeaways
- Simple alkanes exist as a homologous series, in which adjacent members differ by a CH2 unit.
- If the carbon chain of an alkane can branch to make different connections but with the same formula, the different versions are called isomers.
- Because of the non-polar nature of alkanes, they are relatively unreactive; one important exception is combustion with oxygen.

