13.1: Amino Acids
- Page ID
- 58852
Learning Outcomes
- Identify structural components of an amino acid.
- Define zwitterion and isoelectric point.
- Determine the charge on an amino acid when it is not at the isoelectric point.
- Label amino acids as polar and nonpolar and as acidic, basic, or neutral.
Athletics are very competitive these days at all levels, from school sports to the pros. Everybody is looking for that edge that will make them faster, stronger, more physically fit. One approach taken by many athletes is the use of amino acid supplements. The theory is that the increase in amino acids in the diet will lead to increased protein for muscles. However, the only real benefit comes to the people who make and sell the pills. Studies have not showed any advantage obtained by the athletes themselves. You're much better off just maintaining a healthy diet.
Amino Acids
An amino acid is a compound that contains both an amine group \(\left( \ce{-NH_2} \right)\) and a carboxyl group \(\left( \ce{-COOH} \right)\) in the same molecule. While any number of amino acids can possibly be imagined, biochemists generally reserve the term for a group of 20 amino acids which are formed and used by living organisms. The figure below shows the general structure of an amino acid. Either structure is considered correct for an amino acid.

The amine and carboxyl groups of an amino acid are both covalently bonded to a central carbon atom. That carbon atom is also bonded to a hydrogen atom and an \(\ce{R}\) group. It is this \(\ce{R}\) group which varies from one amino acid to another and is called the amino acid side chain.

The nature of the side chains accounts for the variability in physical and chemical properties of the different amino acids. Each amino acid is grouped based on the properties of the side chain. The groups are designated as polar (hydroxylic, sulfur-containing, amidic) , nonpolar (aliphatic and aromatic), acidic, or basic.
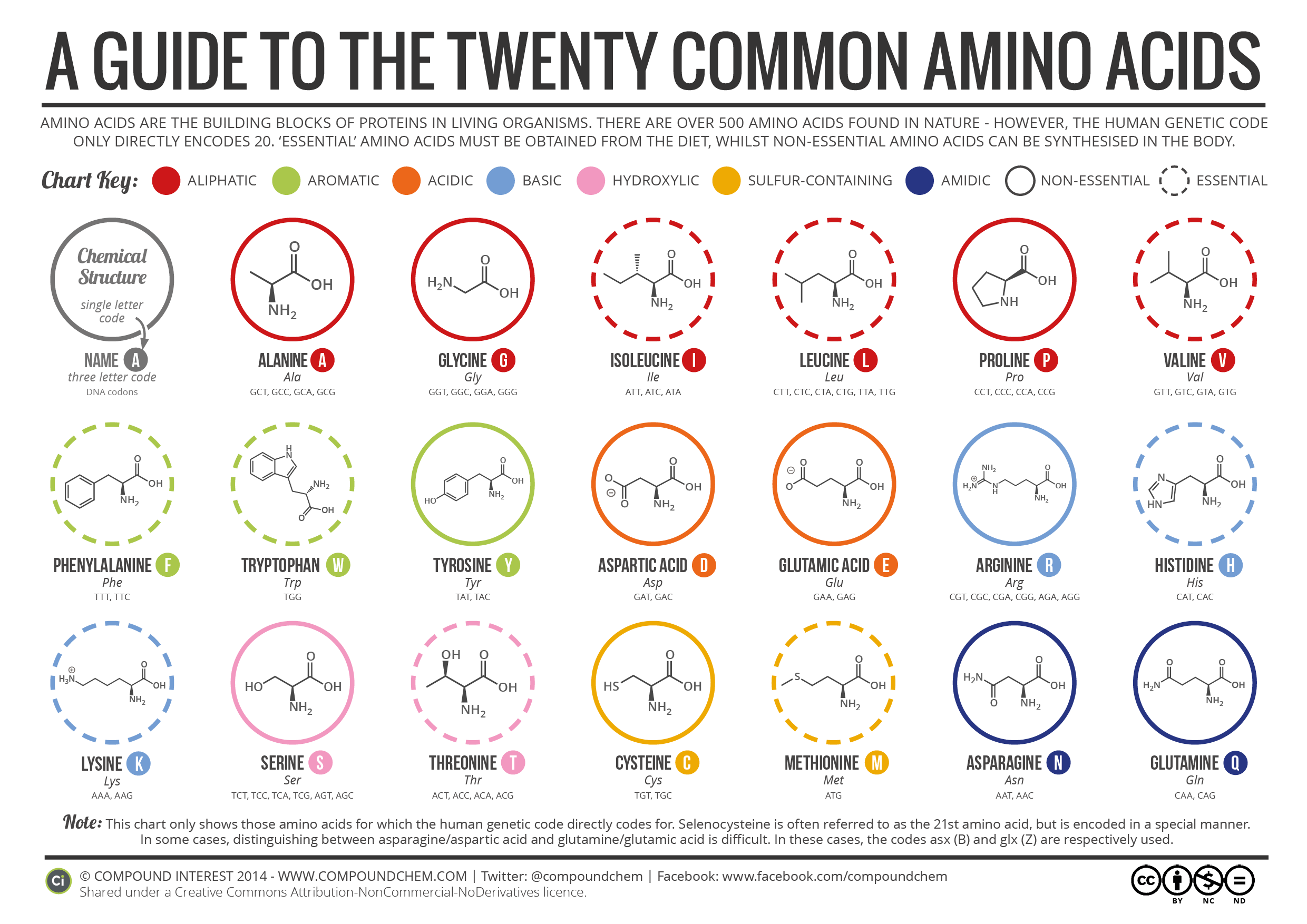
In addition to the full name of the amino acid, there are also one-letter and three-letter abbreviations for each. These abbreviations are especially helpful when listing the amino acids in a protein (a chain of many amino acids that will be discussed later).
Rules for classifying amino acids
The following rules (along with two exceptions) can help you classify amino acids as nonpolar, polar acidic (sometimes called acidic), polar basic (sometimes called basic), or polar neutral. We will look at two exceptions but note that the transition from nonpolar to polar neutral is a gradual transition (like the colors of a rainbow) so you may see variations in how amino acids are classified if you look at other sources.
- Nonpolar amino acids (there are 9) contain aliphatic (hydrocarbon) chains or aromatic rings.
- Polar acidic amino acids (2) contain a carboxylic acid (or carboxylate) group in the side chain (R group). This is in addition to the one in the backbone of the amino acid.
- Polar basic amino acids (3) contain an amine (may be neutral or charged) group in the side chain (R group). This is in addition to the one in the backbone of the amino acid.
- Polar neutral amino acids (6) contain a hydroxyl (-OH), sulfur, or amide in the R group).
There are two important exceptions to the above rules.
- Tyrosine has an aromatic group and an -OH group and is considered polar neutral.
- Methionine contains a sulfur but as a part of carbon chain. Sulfur has the same electronegativity as carbon, so it is considered nonpolar.
Zwitterion
Amino acids are typically drawn either with no charges or with a plus and minus charge (see figure 13.1.1). When an amino acid contains both a plus and a minus charge in the "backbone", it is called a zwitterion and has an overall neutral charge. The zwitterion of an amino acid exists at a pH equal to the isoelectric point. Each amino acid has its own pI value based on the properties of the amino acid. At pH values above or below the isoelectric point, the molecule will have a net charge which depends on its pI value as well as the pH of the solution in which the amino acid is found.
pH < pI
When pH is less than pI, there is an excess amount of \(\ce{H^+}\) in solution. The excess \(\ce{H^+}\) is attracted to the negatively charged carboxylate ion resulting in its protonation. The carbohydrate ion is protonated, making it neutral, leaving only a positive charge on the amine group. Overall, the amino acid will have a charge of \(+1\).

pH > pI
When pH is greater than pI, there is an excess amount of \(\ce{OH^-}\) in solution. The excess \(\ce{OH^-}\) is attracted to the positively charged amine group resulting in the removal of an \(\ce{H^+}\) ion to form (\ce{H_2O}\). The amine group has a neutral charge leaving only a negative charge on the carboxylate group. Overall, the amino acid will have a charge of \(-1\).

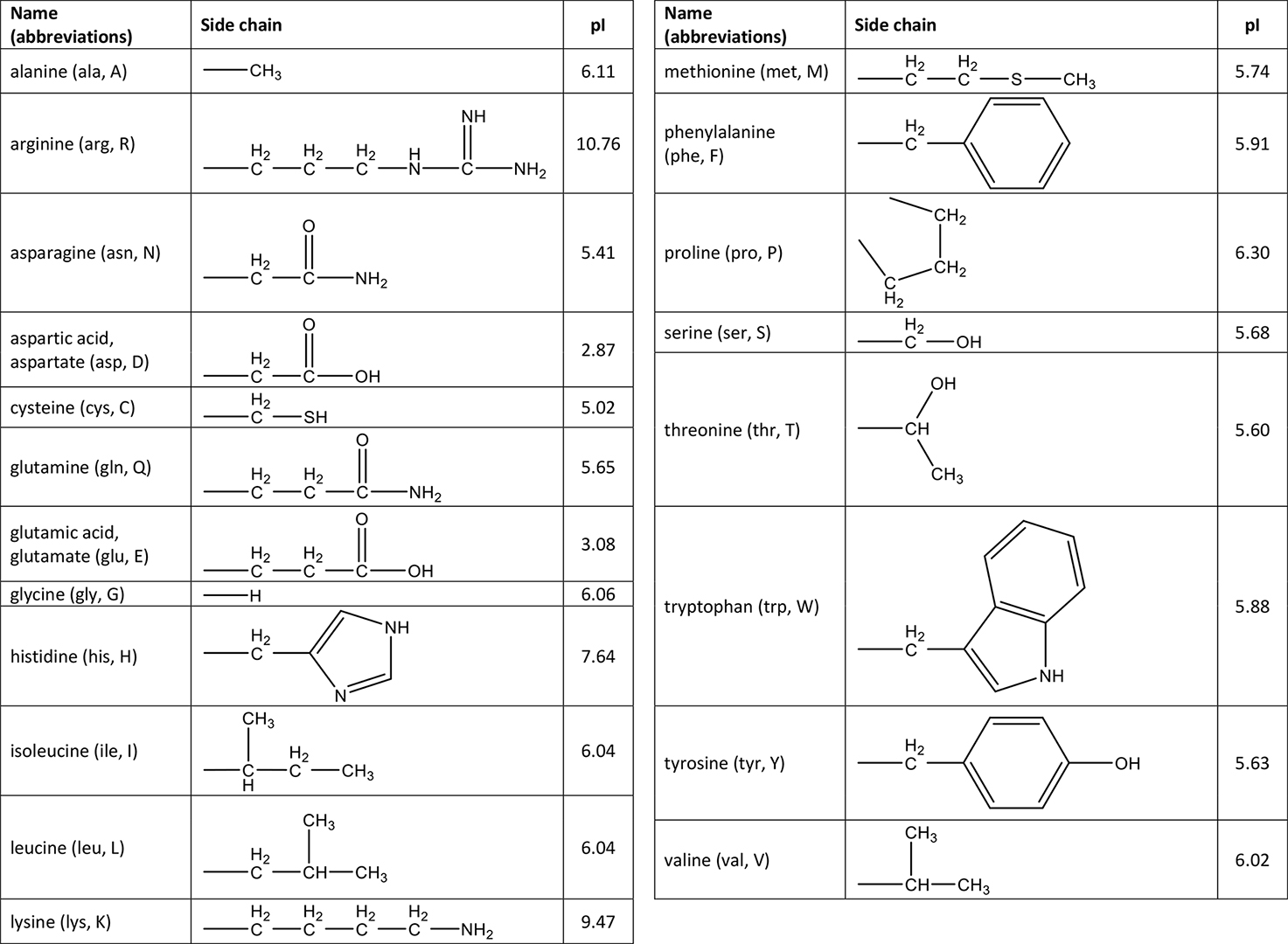
Example \(\PageIndex{1}\)
- Identify the amino acid pictured below.
- Find the pI value for the amino acid.
- Determine how the amino acid will exist at pH = 3.52
- Determine how the amino acid will exist at pH = 9.34
- Determine how the amino acid will exist at pH = 5.02

Solution:
a. Look at the side chain to identify the amino acid. The side chain contains \(\ce{-CH_2SH}\) which matches the structure of cysteine.
b. The pI values for amino acids are found in the table of amino acids. For cysteine, pI = 5.02.
c. At pH = 3.52, the \(\ce{H^+}\) concentration is high (low pH = more acidic = more \(\ce{H^+}\)). Therefore the \(\ce{H^+}\) will add to the carboxylate ion and neutralize the negative charge. The amino acid will have a positive charge on the amine group left and will have an overall charge of \(+1\).

d. At pH = 9.34, the \(\ce{OH^-}\) concentration is high (high pH = more basic = less \(\ce{H^+}\) = more \(\ce{OH^-}\)). Therefore the \(\ce{OH^-}\) will be attracted to the positively charged amine group and will "steal" an \(\ce{H^+}\) from it. As a result, the only remaining charge will be on the carboxylate ion so the amino acid will have a \(-1\) charge.

e. At pH = 5.02, the pH = pI so the amino acid will exist as the zwitterion with both the positive and negative charges as shown above.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)

