2.7: The Periodic Table
- Page ID
- 133344
Skills to Develop
- Explain how elements are organized into the periodic table.
- Describe how some characteristics of elements relate to their positions on the periodic table.
In the 19th century, many previously unknown elements were discovered, and scientists noted that certain sets of elements had similar chemical properties. For example, chlorine, bromine, and iodine react with other elements (such as sodium) to make similar compounds. Likewise, lithium, sodium, and potassium react with other elements (such as oxygen) to make similar compounds. Why is this so?
In 1864, Julius Lothar Meyer, a German chemist, organized the elements by atomic mass and grouped them according to their chemical properties. Later that decade, Dmitri Mendeleev, a Russian chemist, organized all the known elements according to similar properties. He left gaps in his table for what he thought were undiscovered elements, and he made some bold predictions regarding the properties of those undiscovered elements. When elements were later discovered whose properties closely matched Mendeleev’s predictions, his version of the table gained favor in the scientific community. Because certain properties of the elements repeat on a regular basis throughout the table (that is, they are periodic), it became known as the periodic table.
Mendeleev had to list some elements out of the order of their atomic masses to group them with other elements that had similar properties.
The periodic table is one of the cornerstones of chemistry because it organizes all the known elements on the basis of their chemical properties. A modern version is shown in Figure \(\PageIndex{1}\). Most periodic tables provide additional data (such as atomic mass) in a box that contains each element’s symbol. The elements are listed in order of atomic number.
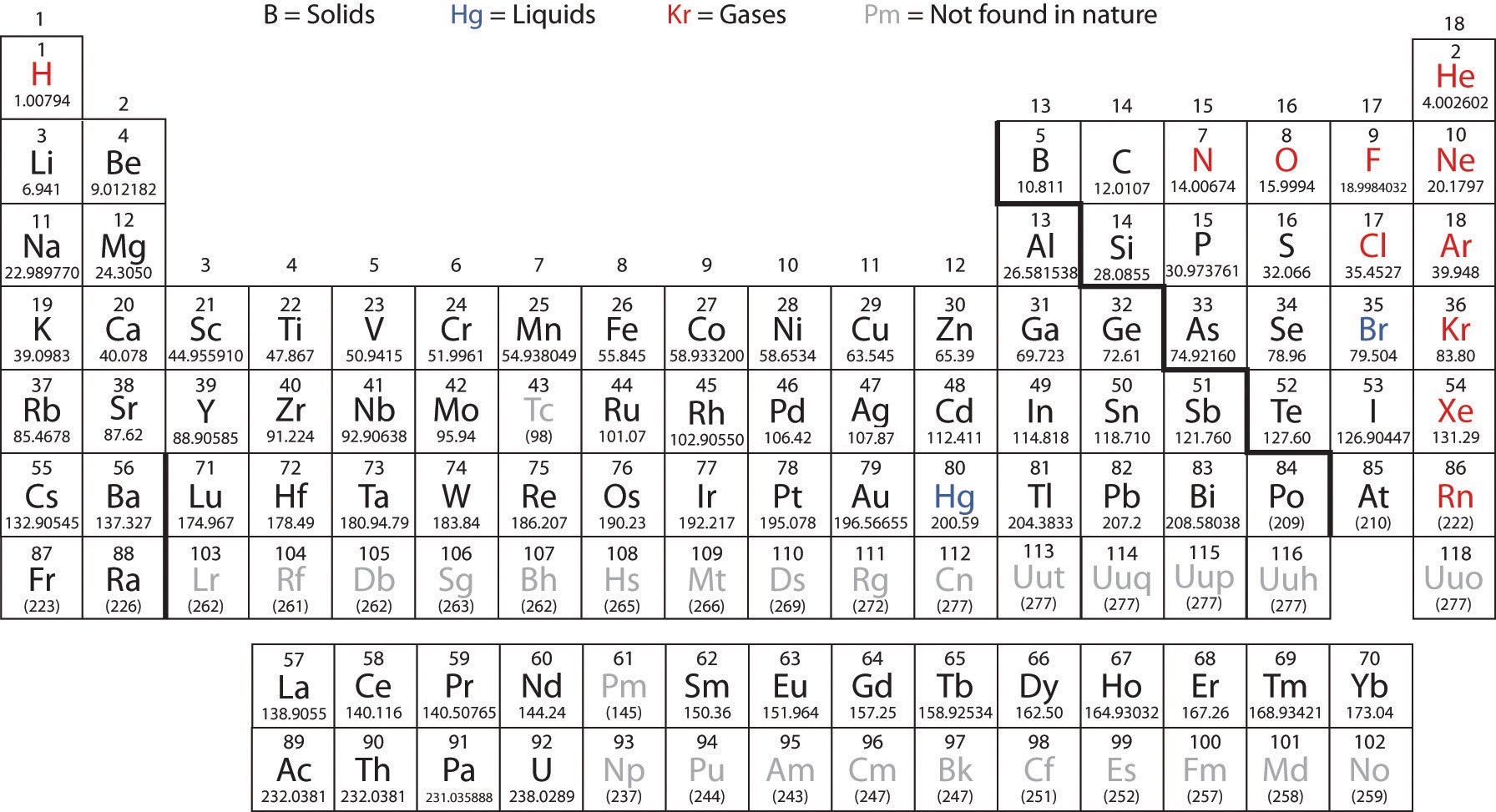
Figure \(\PageIndex{1}\) A Modern Periodic Table. A modern periodic table lists elements left to right by atomic number.
Features of the Periodic Table
Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
The word halogen comes from the Greek for “salt maker” because these elements combine with other elements to form a group of compounds called salts.
To Your Health: Radon
Radon is an invisible, odorless noble gas that is slowly released from the ground, particularly from rocks and soils whose uranium content is high. Because it is a noble gas, radon is not chemically reactive. Unfortunately, it is radioactive, and increased exposure to it has been correlated with an increased lung cancer risk.
Because radon comes from the ground, we cannot avoid it entirely. Moreover, because it is denser than air, radon tends to accumulate in basements, which if improperly ventilated can be hazardous to a building’s inhabitants. Fortunately, specialized ventilation minimizes the amount of radon that might collect. Special fan-and-vent systems are available that draw air from below the basement floor, before it can enter the living space, and vent it above the roof of a house.
After smoking, radon is thought to be the second-biggest preventable cause of lung cancer in the United States. The American Cancer Society estimates that 10% of all lung cancers are related to radon exposure. There is uncertainty regarding what levels of exposure cause cancer, as well as what the exact causal agent might be (either radon or one of its breakdown products, many of which are also radioactive and, unlike radon, not gases). The US Environmental Protection Agency recommends testing every floor below the third floor for radon levels to guard against long-term health effects.
Each row of elements on the periodic table is called a period. Periods have different lengths; the first period has only 2 elements (hydrogen and helium), while the second and third periods have 8 elements each. The fourth and fifth periods have 18 elements each, and later periods are so long that a segment from each is removed and placed beneath the main body of the table.
Certain elemental properties become apparent in a survey of the periodic table as a whole. Every element can be classified as either a metal, a nonmetal, or a semimetal, as shown in Figure \(\PageIndex{2}\). A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). A nonmetal is typically dull and a poor conductor of electricity and heat. Solid nonmetals are also very brittle. As shown in Figure \(\PageIndex{2}\), metals occupy the left three-fourths of the periodic table, while nonmetals (except for hydrogen) are clustered in the upper right-hand corner of the periodic table. The elements with properties intermediate between those of metals and nonmetals are called semimetals (or metalloids). Elements adjacent to the bold line in the right-hand portion of the periodic table have semimetal properties.
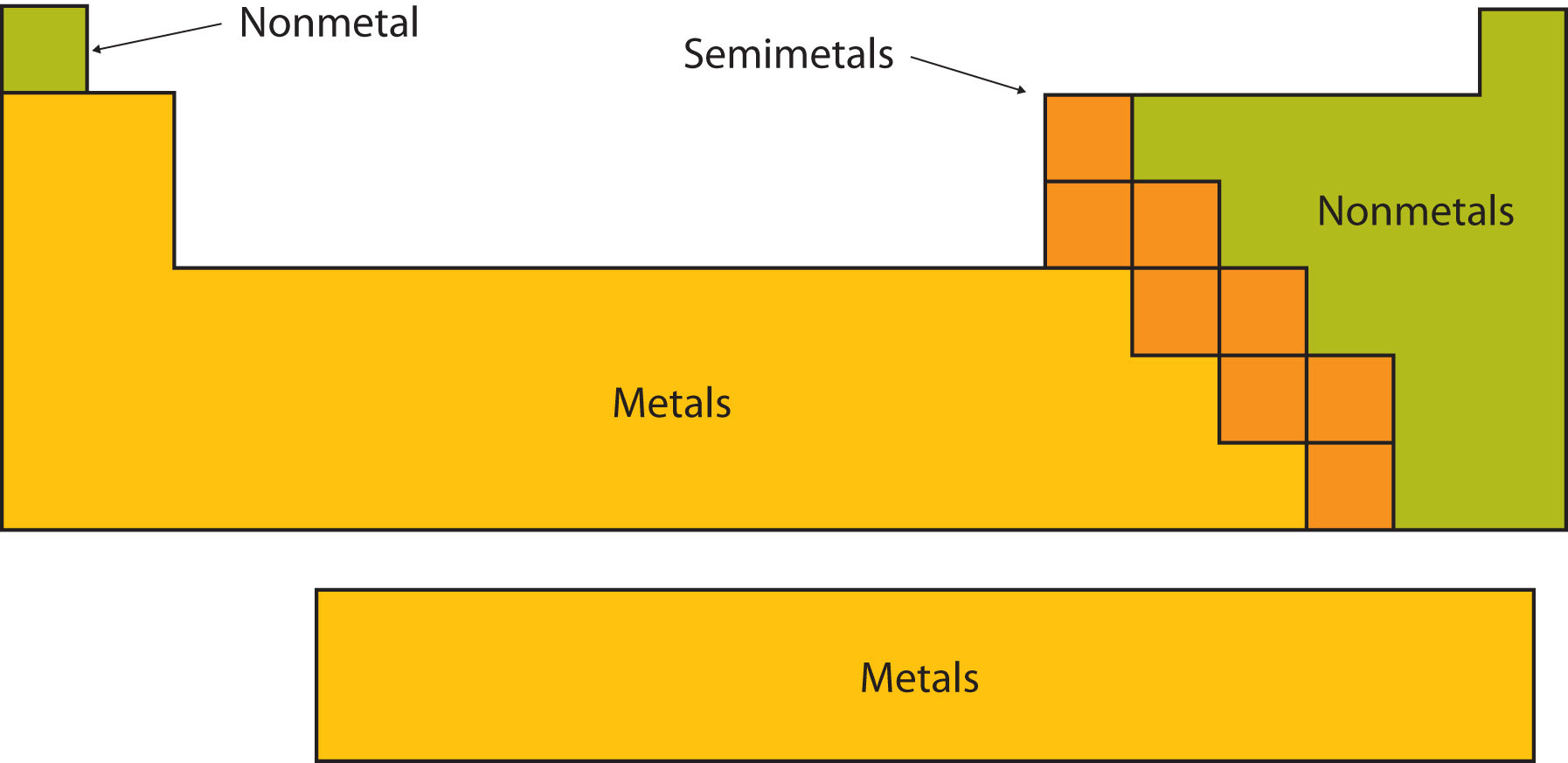
Figure \(\PageIndex{2}\): Types of Elements. Elements are either metals, nonmetals, or semimetals. Each group is located in a different part of the periodic table.
Another way to categorize the elements of the periodic table is shown in Figure \(\PageIndex{3}\). The first two columns on the left and the last six columns on the right are called the main group elements. The ten-column block between these columns contains the transition metals. The two rows beneath the main body of the periodic table contain the inner transition metals. The elements in these two rows are also referred to as, respectively, the lanthanide metals and the actinide metals.
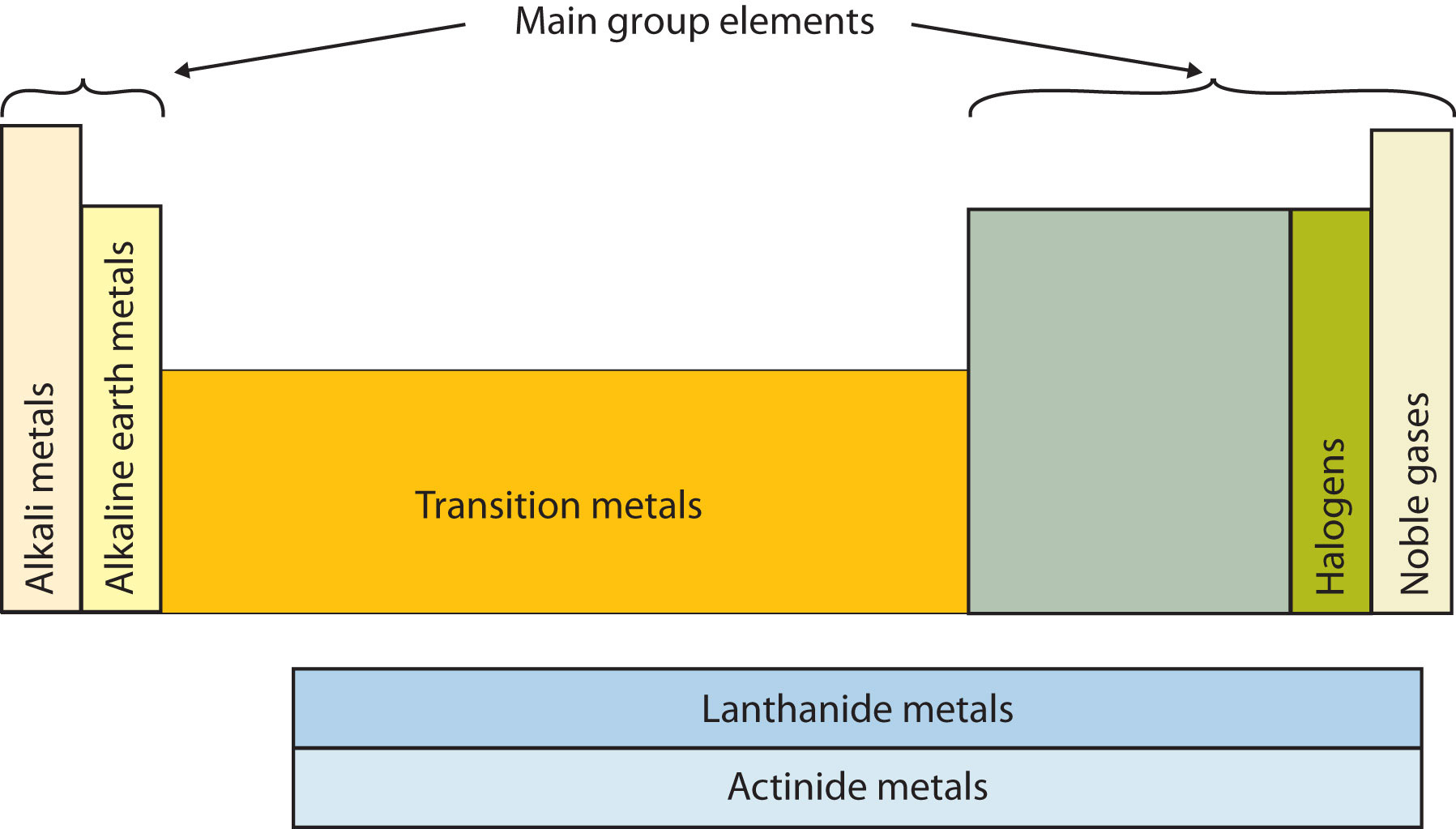
Figure \(\PageIndex{3}\): Special Names for Sections of the Periodic Table. Some sections of the periodic table have special names. The elements lithium, sodium, potassium, rubidium, cesium, and francium are collectively known as alkali metals.
To Your Health: Transition Metals in the Body
According to Table 2.2, most of the elemental composition of the human body consists of main group elements. The first element appearing on the list that is not a main group element is iron, at 0.006 percentage by mass. Because iron has relatively massive atoms, it would appear even lower on a list organized in terms of percent by atoms rather than percent by mass.
Iron is a transition metal. Transition metals have interesting chemical properties, partially because some of their electrons are in d subshells. The chemistry of iron makes it a key component in the proper functioning of red blood cells.
Red blood cells are cells that transport oxygen from the lungs to cells of the body and then transport carbon dioxide from the cells to the lungs. Without red blood cells, animal respiration as we know it would not exist. The critical part of the red blood cell is a protein called hemoglobin. Hemoglobin combines with oxygen and carbon dioxide, transporting these gases from one location to another in the body. Hemoglobin is a relatively large molecule, with a mass of about 65,000 u.
The crucial atom in the hemoglobin protein is iron. Each hemoglobin molecule has four iron atoms, which act as binding sites for oxygen. It is the presence of this particular transition metal in your red blood cells that allows you to use the oxygen you inhale.
Other transition metals have important functions in the body, despite being present in low amounts. Zinc is needed for the body’s immune system to function properly, as well as for protein synthesis and tissue and cell growth. Copper is also needed for several proteins to function properly in the body. Manganese is needed for the body to metabolize oxygen properly. Cobalt is a necessary component of vitamin B-12, a vital nutrient. These last three metals are not listed explicitly in Table 2.2, so they are present in the body in very small quantities. However, even these small quantities are required for the body to function properly.
The periodic table is organized on the basis of similarities in elemental properties, but what explains these similarities? It turns out that the shape of the periodic table reflects the filling of subshells with electrons, as shown in Figure \(\PageIndex{4}\). Starting with the first period and going from left to right, the table reproduces the order of filling of the electron subshells in atoms. Furthermore, elements in the same group share the same valence shell electron configuration. For example, all elements in the first column have a single s electron in their valence shells, so their electron configurations can be described as ns1 (where n represents the shell number). This last observation is crucial. Chemistry is largely the result of interactions between the valence electrons of different atoms. Thus, atoms that have the same valence shell electron configuration will have similar chemistry.
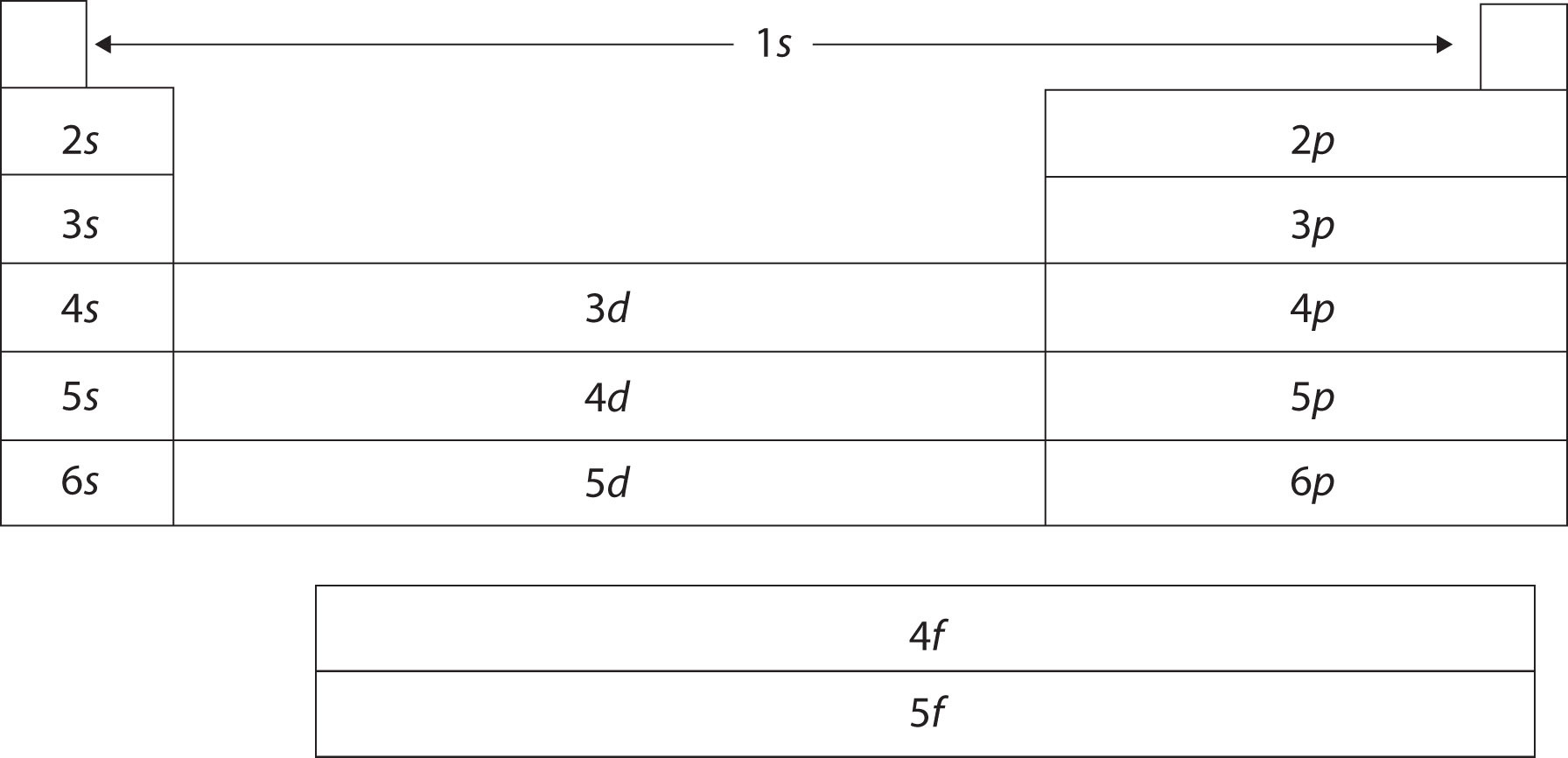
Figure \(\PageIndex{4}\): The Shape of the Periodic Table. The shape of the periodic table reflects the order in which electron shells and subshells fill with electrons.
Example \(\PageIndex{1}\)
Using the variable n to represent the number of the valence electron shell, write the valence shell electron configuration for each group.
- the alkaline earth metals
- the column of elements headed by carbon
- Answer a
-
The alkaline earth metals are in the second column of the periodic table. This column corresponds to the s subshell being filled with 2 electrons. Therefore, the valence shell electron configuration is ns2.
- Answer b
-
The electron configuration of carbon is 1s22s22p2. Its valence shell electron configuration is 2s22p2. Every element in the same column should have a similar valence shell electron configuration, which we can represent as ns2np2.
Exercise \(\PageIndex{1}\)
Using the variable n to represent the number of the valence electron shell, write the valence shell electron configuration for each group.
- the halogens
- the column of elements headed by oxygen
Atomic Radius
The periodic table is useful for understanding atomic properties that show periodic trends. One such property is the atomic radius (Figure \(\PageIndex{5}\)). As mentioned earlier, the higher the shell number, the farther from the nucleus the electrons in that shell are likely to be. In other words, the size of an atom is generally determined by the number of the valence electron shell. Therefore, as we go down a column on the periodic table, the atomic radius increases. As we go across a period on the periodic table, however, electrons are being added to the same valence shell; meanwhile, more protons are being added to the nucleus, so the positive charge of the nucleus is increasing. The increasing positive charge attracts the electrons more strongly, pulling them closer to the nucleus. Consequently, as we go across a period, the atomic radius decreases. These trends are seen clearly in Figure \(\PageIndex{5}\).
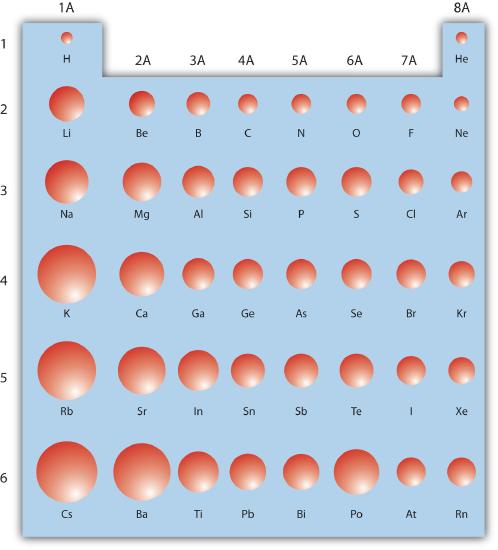
Figure \(\PageIndex{5}\): Trends on the Periodic Table. The relative sizes of the atoms show several trends with regard to the structure of the periodic table. Atoms become larger going down a column and smaller going across a period.
Example \(\PageIndex{2}\)
Using the periodic table (rather than Figure \(\PageIndex{5}\)), which atom is larger?
- N or Bi
- Mg or Cl
- Answer a
-
Because Bi is below N on the periodic table and has electrons in higher-numbered shells, we expect that Bi atoms are larger than N atoms.
- Answer b
-
Both Mg and Cl are in period 3 of the periodic table, but Cl lies farther to the right. Therefore we expect Mg atoms to be larger than Cl atoms.
Exercise \(\PageIndex{2}\)
Using the periodic table (rather than Figure \(\PageIndex{5}\)), which atom is larger?
- Li or F
- Na or K
Career Focus: Clinical Chemist
Clinical chemistry is the area of chemistry concerned with the analysis of body fluids to determine the health status of the human body. Clinical chemists measure a variety of substances, ranging from simple elements such as sodium and potassium to complex molecules such as proteins and enzymes, in blood, urine, and other body fluids. The absence or presence, or abnormally low or high amounts, of a substance can be a sign of some disease or an indication of health. Many clinical chemists use sophisticated equipment and complex chemical reactions in their work, so they not only need to understand basic chemistry, but also be familiar with special instrumentation and how to interpret test results.
Concept Review Exercises
-
How are the elements organized into the periodic table?
-
Looking at the periodic table, where do the following elements appear?
- the metals
- the nonmetals
- the halogens
- the transition metals
-
Describe the trends in atomic radii as related to an element’s position on the periodic table.
Answers
-
Elements are organized by atomic number.
-
- the left three-quarters of the periodic table
- the right quarter of the periodic table
- the next-to-last column of the periodic table
- the middle section of the periodic table
-
As you go across the periodic table, atomic radii decrease; as you go down the periodic table, atomic radii increase.
Key Takeaways
- The chemical elements are arranged in a chart called the periodic table.
- Some characteristics of the elements are related to their position on the periodic table.

