Dihydroxylation of alkenes
Alkenes can be dihydroxylated by two different stereochemical pathways: anti-dihydroxylation or syn-dihydroxylation. The opening of epoxides follows the anti-dihydroxylation mechanism, while potassium permanganate or osmium tetroxide produce the syn-dihydroxylated products. The osmium tertroxide reaction can also take place by a two-step process: 1) OsO4 in pyridine followed by 2) H2S or NaHSO3. It is important to note that different professors will emphasize different reagent systems to accomplish the same chemical reaction. In these situations, it can be helpful to recognize the role of each reagent to discern patterns.
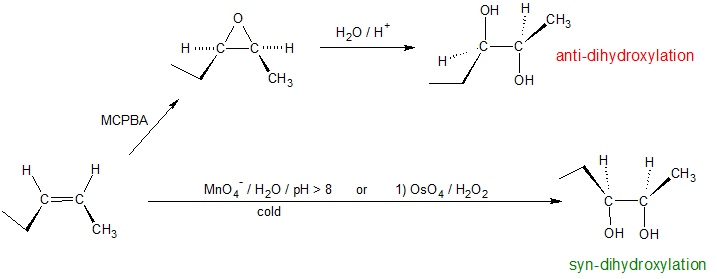
Anti Dihydroxylation
Epoxides may be cleaved by aqueous acid to give glycols that are often diastereomeric with those prepared by the syn-hydroxylation reaction described above. Proton transfer from the acid catalyst generates the conjugate acid of the epoxide, which is attacked by nucleophiles such as water in the same way that the cyclic bromonium ion described above undergoes reaction. The result is anti-hydroxylation of the double bond, in contrast to the syn-stereoselectivity of the earlier method. In the following equation this procedure is illustrated for a cis-disubstituted epoxide, which, of course, could be prepared from the corresponding cis-alkene. This hydration of an epoxide does not change the oxidation state of any atoms or groups. The mechanism for the ring opening of epoxides depends on the reaction conditions and is discussed in more detail in the next section of this chapter.
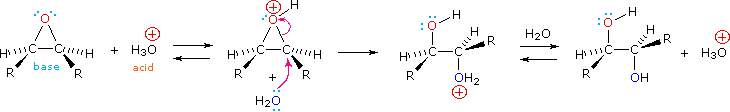
Syn Dihydroxylation
Osmium tetroxide oxidizes alkenes to give glycols through syn addition. A glycol, also known as a vicinal diol, is a compound with two -OH groups on adjacent carbons.
Dihydroxylated products (glycols) are obtained by reaction with aqueous potassium permanganate (pH > 8) or osmium tetroxide in pyridine solution. Both reactions appear to proceed by the same mechanism (shown below); the metallocyclic intermediate may be isolated in the osmium reaction. In basic solution the purple permanganate anion is reduced to the green manganate ion, providing a nice color test for the double bond functional group. From the mechanism shown here we would expect syn-stereoselectivity in the bonding to oxygen, and regioselectivity is not an issue.
When viewed in context with the previously discussed addition reactions, the hydroxylation reaction might seem implausible. Permanganate and osmium tetroxide have similar configurations, in which the metal atom occupies the center of a tetrahedral grouping of negatively charged oxygen atoms. How, then, would such a species interact with the nucleophilic pi-electrons of a double bond? A possible explanation is that an empty d-orbital of the electrophilic metal atom extends well beyond the surrounding oxygen atoms and initiates electron transfer from the double bond to the metal, in much the same fashion noted above for platinum. Back-bonding of the nucleophilic oxygens to the antibonding π*-orbital completes this interaction. The result is formation of a metallocyclic intermediate, as shown above.
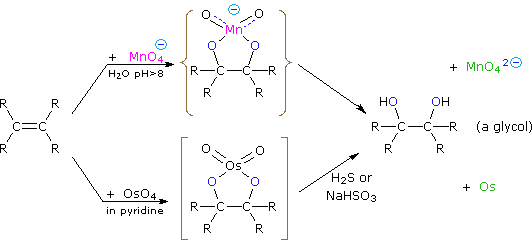
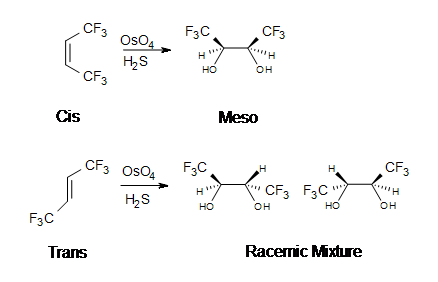
The reaction with \(OsO_4\) is a concerted process that has a cyclic intermediate and no rearrangements. Vicinal syn dihydroxylation complements the epoxide-hydrolysis sequence which constitutes an anti dihydroxylation of an alkene. When an alkene reacts with osmium tetroxide, stereocenters can form in the glycol product. Cis alkenes give meso products and trans alkenes give racemic mixtures.
\(OsO_4\) is formed slowly when osmium powder reacts with gasoues \(O_2\) at ambient temperature. Reaction of bulk solid requires heating to 400 °C:
\[Os_{(s)} + 2O_{2\;(g)} \rightarrow OS_4\]
Since Osmium tetroxide is expensive and highly toxic, the reaction with alkenes has been modified. Catalytic amounts of OsO4 and stoichiometric amounts of an oxidizing agent such as hydrogen peroxide are now used to eliminate some hazards. Also, an older reagent that was used instead of OsO4 was potassium permanganate, \(KMnO_4\). Although syn diols will result from the reaction of KMnO4 and an alkene, potassium permanganate is less useful since it gives poor yields of the product because of overoxidation.
Chemical Highlight
Antitumor drugs have been formed by using dihydroxylation. This method has been applied to the enantioselective synthesis of ovalicin, which is a class of fungal-derived products called antiangiogenesis agents. These antitumor products can cut off the blood supply to solid tumors. A derivative of ovalicin, TNP-470, is chemically stable, nontoxic, and noninflammatory. TNP-470 has been used in research to determine its effectiveness in treating cancer of the breast, brain, cervix, liver, and prostate.
Exercise
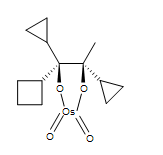
1. Give the major product.
.bmp?revision=1&size=bestfit&width=131&height=51)
2. What is the product in the dihydroxylation of (Z)-3-hexene?

3. What is the product in the dihydroxylation of (E)-3-hexene?

4. Draw the intermediate of this reaction.

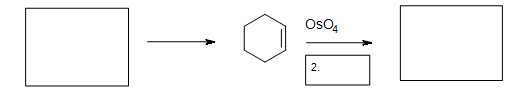
5. Fill in the missing reactants, reagents, and product.
- Answer
-
1. A syn-1,2-ethanediol is formed. There is no stereocenter in this particular reaction. The OH groups are on the same side.
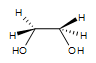
2. Meso-3,4-hexanediol is formed. There are 2 stereocenters in this reaction.
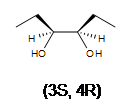
3. A racemic mixture of 3,4-hexanediol is formed. There are 2 stereocenters in both products.

4. A cyclic osmic ester is formed.
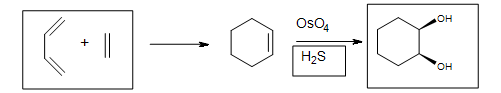
5. The Diels-Alder cycloaddition reaction is needed in the first box to form the cyclohexene. The second box needs a reagent to reduce the intermediate cyclic ester (not shown). The third box has the product: 1,2-cyclohexanediol.
References
- Dehestani, Ahmad et al. (2005). Ligand-assisted reduction of osmium tetroxide with molecular hydrogen via a [3+2] mechanism. Journal of the American Chemical Society, 2005, 127 (10), 3423-3432.
- Sorrell, Thomas, N. Organic Chemistry. New York: University Science Books, 2006.
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
Contributors and Attributions



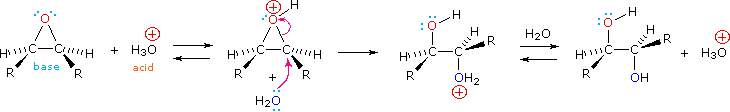



.bmp?revision=1&size=bestfit&width=131&height=51)








