18.5 Friedel–Crafts Alkylation and Acylation
- Page ID
- 28352
Friedel-Crafts Alkylation
Friedel-Crafts alkylation is accomplished by reacting an aromatic ring with an alkyl halide in the presence of an appropriate Lewis Acid catalyst as shown in the following mechanism.
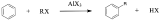
The mechanism takes place as follows:
The reactivity of haloalkanes increases as you move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl then RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions.
Some limitations of Friedel-Crafts Alkylation
There are possibilities of carbocation rearrangements when you are trying to add a carbon chain greater than two carbons. The rearrangements occur due to hydride shifts and methyl shifts. For example, the product of a Friedel-Crafts Alkylation will show an iso rearrangement when adding a three carbon chain as a substituent. Also, the reaction will only work if the ring you are adding a substituent to is not deactivated. For a look at substituents that activate or deactivate a benzene ring, check out the wiki page: Activating and Deactivating Benzene Rings One way to resolve these problems is through Friedel-Crafts Acylation.
Friedel-Crafts Alkylation was first discovered by French scientist Charles Friedel and his partner, American scientist James Crafts, in 1877. This reaction allowed for the formation of alkyl benzenes from alkyl halides, but was plagued with unwanted supplemental activity that reduced its effectivity.
Limitations of Friedel-Crafts Alkylation
- Carbocation Rearrangement - Only certain alkylbenzenes can be made due to the tendency of cations to rearrange.
- Compound Limitations - Friedel-Crafts fails when used with compounds such as nitrobenzene and other strong deactivating systems.
- Polyalkylation - Products of Friedel-Crafts are even more reactive than starting material. Alkyl groups produced in Friedel-Crafts Alkylation are electron-donating substituents meaning that the products are more susceptible to electrophilic attack than what we began with. For synthetic purposes, this is a big dissapointment.
To remedy these limitations, a new and improved reaction was devised: The Friedel-Crafts Acylation, also known as Friedel-Crafts Alkanoylation
Friedel-Crafts Acylation
Friedel-Crafts acylation follows a similar mechanism as the alkylation with the first step being activation of the acyl halide. The resulting product places a carbonyl group next to the aromatic ring.
The goal of the reaction is the following:
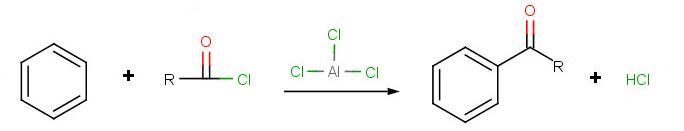.jpg?revision=1)
The very first step involves the formation of the acylium ion which will later react with benzene:
The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:
.jpg?revision=1)
The third step involves the departure of the proton in order for aromaticity to return to benzene:
During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. Thie first part of the product is the complex with aluminum chloride as shown:
.jpg?revision=1)
The final step involves the addition of water to liberate the final product as the acylbenzene:
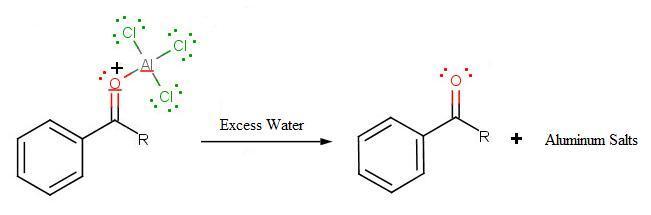.jpg?revision=1)
Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (Limitation 1). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts acylation fails with strong deactivating rings.
Contributors
- Layne A. Morsch - University of Illinois Springfield



.bmp?revision=1&size=bestfit&width=720&height=414)
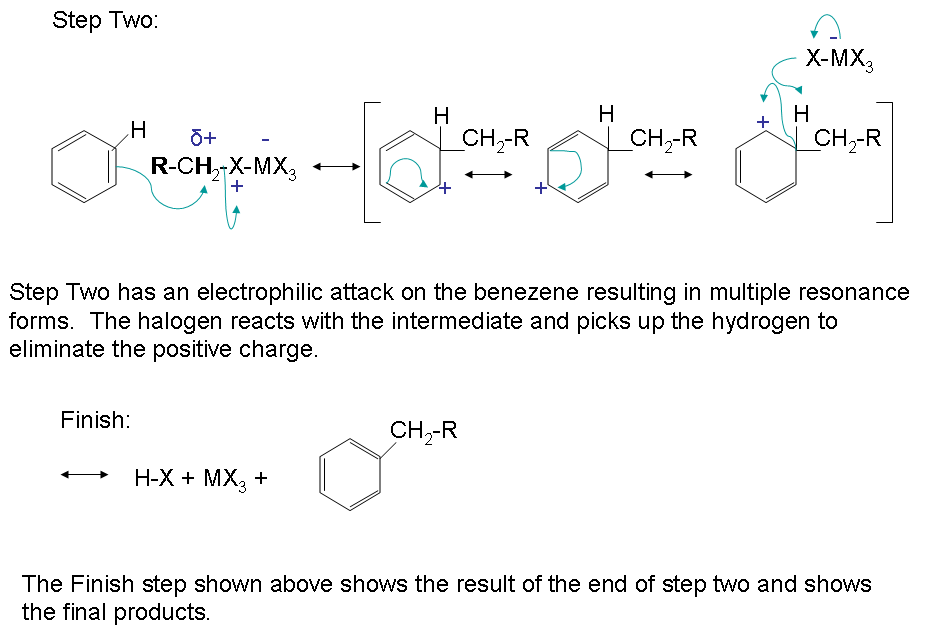
.jpg?revision=1&size=bestfit&width=720&height=114)
.jpg?revision=1)