9.1: Introduction to Alcohols, Ethers and Epoxides
- Page ID
- 28195
We have already seen the simplest possible example of an alcohol functional group in methanol. In the alcohol functional group, a carbon is single-bonded to an OH group (this OH group, by itself, is referred to as a hydroxyl). If the central carbon in an alcohol is bonded to only one other carbon, we call the group a primary alcohol. In secondary alcohols and tertiary alcohols, the central carbon is bonded to two and three carbons, respectively. Methanol, of course, is in class by itself in this respect.

Primary alcohols
In a primary (1°) alcohol, the carbon atom that carries the -OH group is only attached to one alkyl group. Some examples of primary alcohols are shown below:
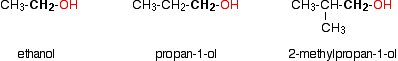
Notice that the complexity of the attached alkyl group is irrelevant. In each case there is only one linkage to an alkyl group from the CH2 group holding the -OH group. There is an exception to this. Methanol, CH3OH, is counted as a primary alcohol even though there are no alkyl groups attached to the the -OH carbon atom.
Secondary alcohols
In a secondary (2°) alcohol, the carbon atom with the -OH group attached is joined directly to two alkyl groups, which may be the same or different. Examples include the following:
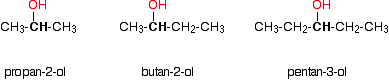
Tertiary alcohols
In a tertiary (3°) alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of the same or different groups. Examples of tertiary alcohols are given below:
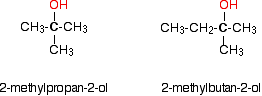 '
'
Ethers
With the general formula ROR′, an ether may be considered a derivative of water in which both hydrogen atoms are replaced by alkyl or aryl groups. It may also be considered a derivative of an alcohol (ROH) in which the hydrogen atom of the OH group is been replaced by a second alkyl or aryl group:
\(\mathrm{HOH\underset{H\: atoms}{\xrightarrow{replace\: both}}ROR'\underset{of\: OH\: group}{\xleftarrow{replace\: H\: atom}}ROH}\)
Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. For example, CH3–O–CH2CH2CH3 is methyl propyl ether. If both groups are the same, the group name should be preceded by the prefix di-, as in dimethyl ether (CH3–O–CH3) and diethyl ether CH3CH2–O–CH2CH3.
Epoxides
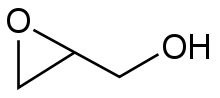
An epoxide is a cyclic ether with three ring atoms. These rings approximately define an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in chloromethyloxirane. As a functional group, epoxides feature the epoxy prefix, such as in the compound 1,2-epoxycycloheptane, which can also be called cycloheptene epoxide, or simply cycloheptene oxide.

A generic epoxide.
The chemical structure of the epoxide glycidol, a common chemical intermediate
A polymer formed by reacting epoxide units is called a polyepoxide or an epoxy. Epoxy resins are used as adhesives and structural materials. Polymerization of an epoxide gives a polyether, for example ethylene oxide polymerizes to give polyethylene glycol, also known as polyethylene oxide.
Contributors
Jim Clark (Chemguide.co.uk)
- Wikipedia