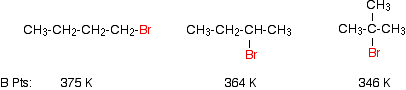7.3: Physical Properties of Alkyl Halides
- Page ID
- 28166
Physical properties of alkyl halides
Boiling Points
The chart shows the boiling points of some simple alkyl halides.
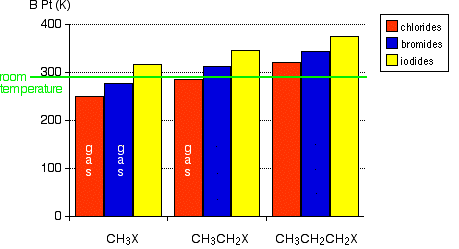
Notice that three of these have boiling points below room temperature (taken as being about 20°C). These will be gases at room temperature. All the others you are likely to come across are liquids. Remember:
- the only methyl halide which is a liquid is iodomethane;
- chloroethane is a gas.
The patterns in boiling point reflect the patterns in intermolecular attractions.
van der Waals dispersion forces
These attractions get stronger as the molecules get longer and have more electrons. That increases the sizes of the temporary dipoles that are set up. This is why the boiling points increase as the number of carbon atoms in the chains increases. Look at the chart for a particular type of halide (a chloride, for example). Dispersion forces get stronger as you go from 1 to 2 to 3 carbons in the chain. It takes more energy to overcome them, and so the boiling points rise.
The increase in boiling point as you go from a chloride to a bromide to an iodide (for a given number of carbon atoms) is also because of the increase in number of electrons leading to larger dispersion forces. There are lots more electrons in, for example, iodomethane than there are in chloromethane - count them!
van der Waals dipole-dipole attractions
The carbon-halogen bonds (apart from the carbon-iodine bond) are polar, because the electron pair is pulled closer to the halogen atom than the carbon. This is because (apart from iodine) the halogens are more electronegative than carbon. The electronegativity values are:
| C | 2.5 | F | 4.0 | |
| Cl | 3.0 | |||
| Br | 2.8 | |||
| I | 2.5 |
This means that in addition to the dispersion forces there will be forces due to the attractions between the permanent dipoles (except in the iodide case). The size of those dipole-dipole attractions will fall as the bonds get less polar (as you go from chloride to bromide to iodide, for example). Nevertheless, the boiling points rise! This shows that the effect of the permanent dipole-dipole attractions is much less important than that of the temporary dipoles which cause the dispersion forces. The large increase in number of electrons by the time you get to the iodide completely outweighs the loss of any permanent dipoles in the molecules.
| Example 1: Boiling Points of Some Isomers |
|---|
| The examples show that the boiling points fall as the isomers go from a primary to a secondary to a tertiary halogenoalkane. This is a simple result of the fall in the effectiveness of the dispersion forces.
The temporary dipoles are greatest for the longest molecule. The attractions are also stronger if the molecules can lie closely together. The tertiary halogenoalkane is very short and fat, and won't have much close contact with its neighbours. |
Solubility
Solubility in water
The alkyl halides are at best only slightly soluble in water. For a halogenoalkane to dissolve in water you have to break attractions between the halogenoalkane molecules (van der Waals dispersion and dipole-dipole interactions) and break the hydrogen bonds between water molecules. Both of these cost energy.
Energy is released when new attractions are set up between the halogenoalkane and the water molecules. These will only be dispersion forces and dipole-dipole interactions. These aren't as strong as the original hydrogen bonds in the water, and so not as much energy is released as was used to separate the water molecules. The energetics of the change are sufficiently "unprofitable" that very little dissolves.
Solubility in organic solvents
alkyl halides tend to dissolve in organic solvents because the new intermolecular attractions have much the same strength as the ones being broken in the separate halogenoalkane and solvent.
Chemical Reactivity
The pattern in strengths of the four carbon-halogen bonds are:
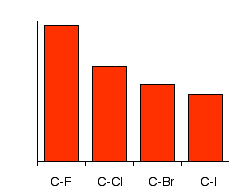
Notice that bond strength falls as you go from C-F to C-I, and notice how much stronger the carbon-fluorine bond is than the rest. To react with the alkyl halides, the carbon-halogen bond has got to be broken. Because that gets easier as you go from fluoride to chloride to bromide to iodide, the compounds get more reactive in that order. Iodoalkanes are the most reactive and fluoroalkanes are the least. In fact, fluoroalkanes are so unreactive that we shall pretty well ignore them completely from now on in this section!
Contributors
Jim Clark (Chemguide.co.uk)